Ionic Polar Covalent Nonpolar Covalent Chart
Examples of Molecules with Polar Covalent Bonds. Atoms with similar EN Polar Covalent Bonds.
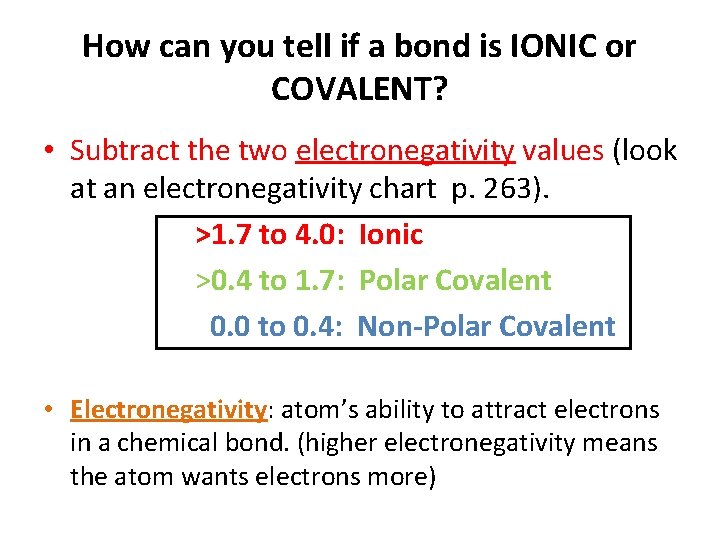
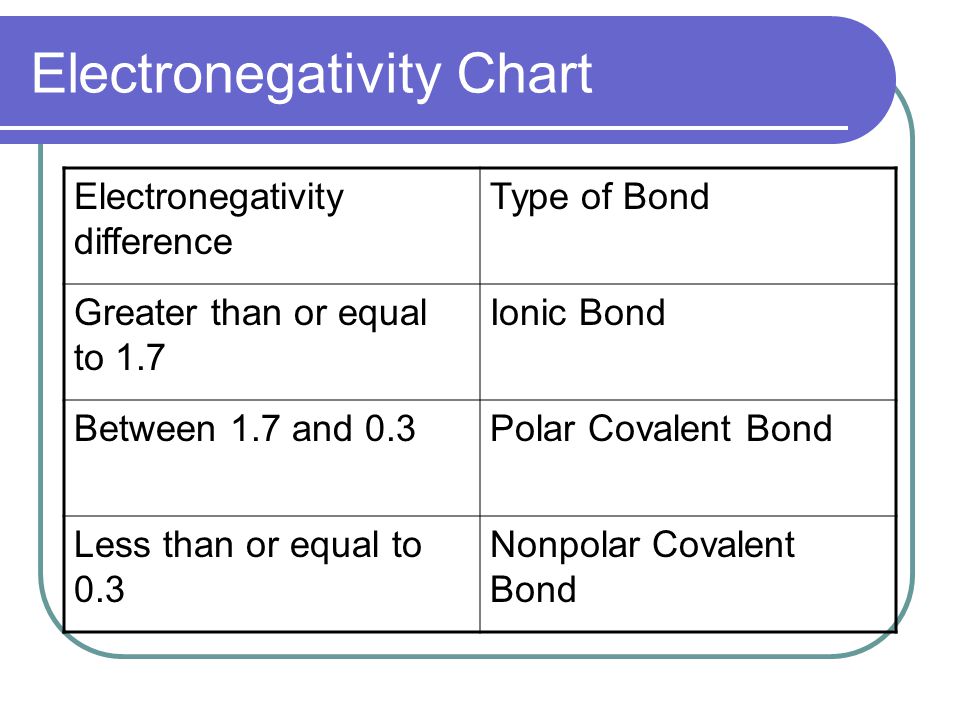
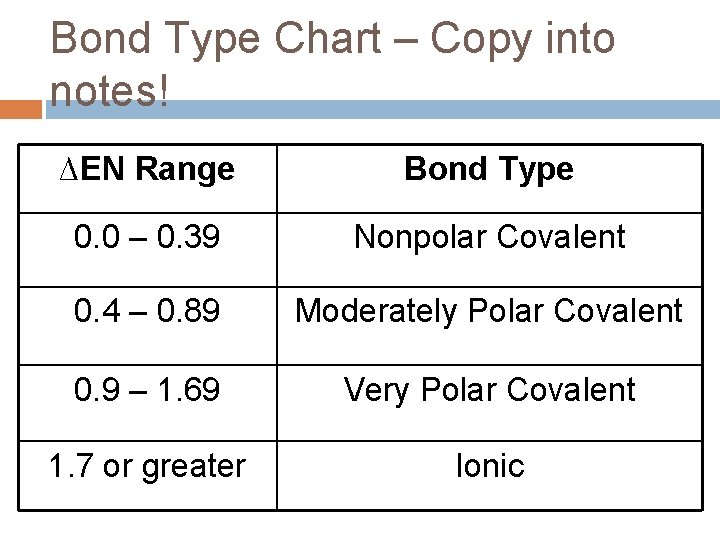
Covalent Bonding Notes Ionic Vs Covalent Bonding Ionic
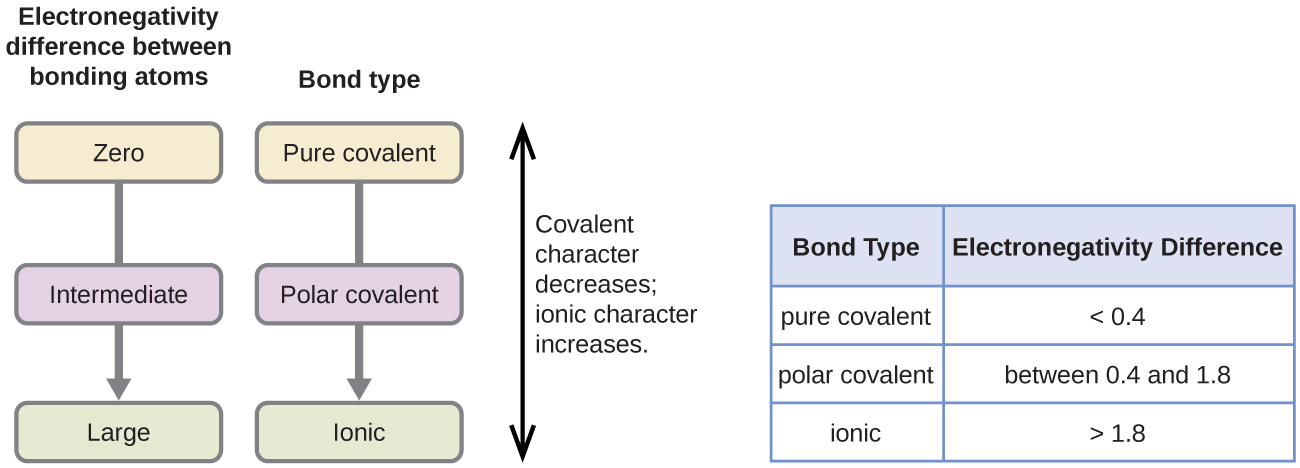
You have to calculate the difference in electronegativities between the atoms.

Ionic polar covalent nonpolar covalent chart. Hello the true choice from the one you gave us is that a bond formed between a silicon atom and an oxygen atom is likely to be B. Polar Covalent Bonds Polar covalent bonding is a type of chemical bonding where a pair of electrons is unequally shared between two atoms. Neither atom completely loses or gains electrons as in ionic bonding.
Difference in EN 2 CH bonds relatively nonpolar C-O C-X bonds more electronegative elements are polarelectronegative elements are polar Bonding electrons shift toward electronegative atom. 1 Polar versus Nonpolar Covalent Bonds. Non-polar bonding with an equal sharing of electrons.
This is a nonpolar covalent bond. The rule is that when the electronegativity level is greater than 2 the bond is considered ionic. We need this chart when we are trying to decipher whether a molecule is polar or nonpolar in nature.
Also Read What is Nonpolar Covalent Bond. Some elements tend to attract electrons more strongly than others. This video is a brief lesson about three types of chemical bonding--nonpolar covalent polar covalent and ionic bonding and how to predict which type of bo.
When ΔEN is less than 05 the. In other words the distribution of electrons around the molecule is no longer balanced. Formulas For Binary Ionic Compounds Practice By Integrationscience Teaching Resources Ionic.
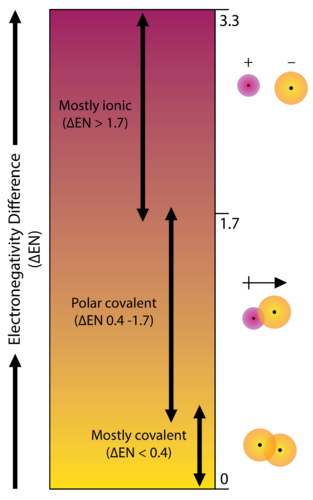
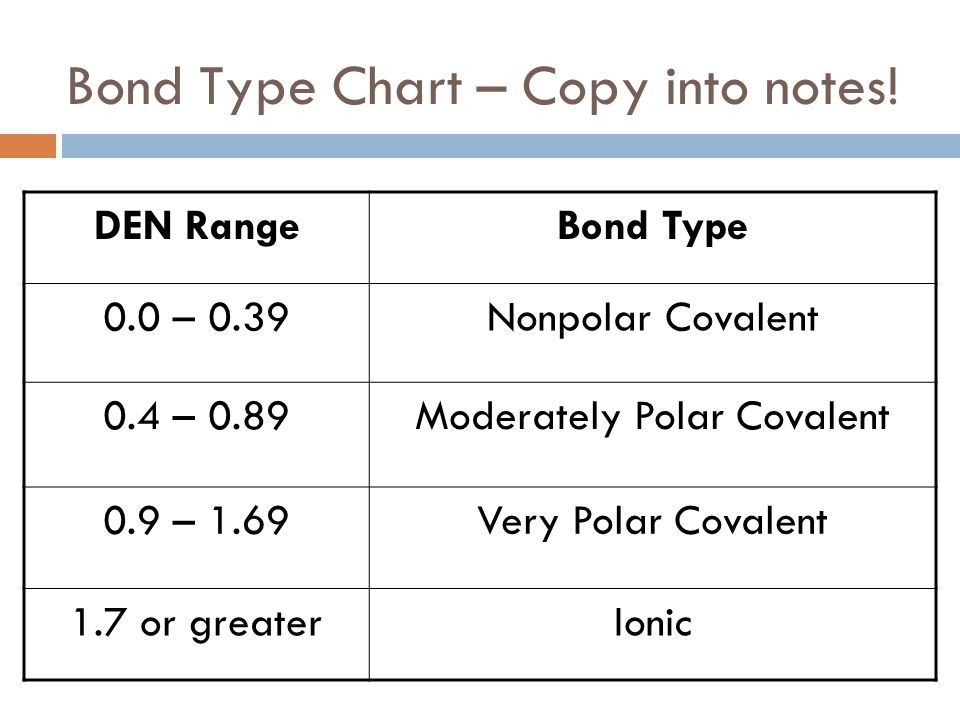
Electrons are shared differently in ionic and covalent bonds. Polar covalent bonds shape among atoms with an electronegativity distinction among 0four and 17. This is a nonpolar covalent bond.
Difference in EN of atoms 2 Ionic Bonds. This is a polar covalent bond. In polar covalent bonds the bond is formed between atoms with different electronegativities for example H2O HCl and NH3 whereas in nonpolar covalent bonds it is made up of identical atoms.
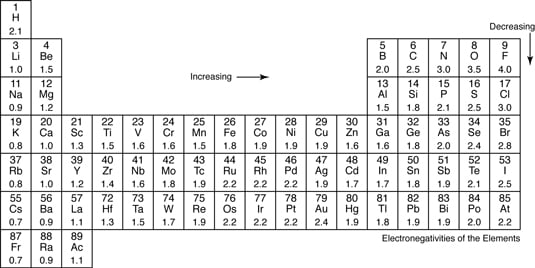
Ionic bonds like those in table salt NaCl are due to electrostatic attractive forces between their positive Na and negative charged Cl- ions. How to tell if a bond is Ionic Covalent or Polar Covalent. Electronegativity Polar Covalent Bonds.
Electronegativity is the tendency of an atom to attract a. Atoms with high. Bond Polarity and Inductive EffectBond Polarity and Inductive Effect Nonpolar Covalent Bonds.
A The electrons in the covalent bond are equally shared by both hydrogen atoms. In general let x be the difference in electronegativity between bonding atoms. Most compounds contain polar covalent bonds.
In covalent bonding the two electrons shared by the atoms are attracted to the nucleus of both atoms. As discussed in Module 5 to determine whether or not a molecule is polar covalent or nonpolar covalent you. This type of bonding is actually somewhere between the nonpolar covalent bonds and ionic bonding.
Teacherlingo Com 1 99 Students Fill In The Chart For Ionic Covalent Polar And Nonpolar And Metallic Bonds Chemical Bond Science Resources Chart. This is due to one of the elements having a higher electronegativity than the other. Covalent bonds can be non-polar or polar and react to electrostatic charges.
If there are only non-metals right side of the jagged line on the periodic chart it is a covalent compound. When the level is less than5 it is a non-polar covalent bond. The difference in electronegativities of the atoms joined by a chemical bond allows us to classify the type of bond.
B The chlorine atom attracts the electrons in the bond more than the hydrogen atom does leading to an imbalance in the electron distribution. High boiling and melting points. Nonpolar covalent bonds have low boiling points and melting points whereas polar covalent bonds have a high boiling point and melting point.
When two or more atoms share electrons the bond is referred to as A. Polar covalentThank you Marvel2Points 4929. According to the generalized notion when the electronegativity difference between two atoms in a bond is less than 04-05 it is said to be a nonpolar covalent bond.
The electronegativity cost of oxygen is 344 at the same time as the electronegativity of hydrogen is 220. Water H2O is a polar bond molecule. B The fluorine atom attracts the electrons in the bond more than the hydrogen atom does leading to an imbalance in the electron distribution.
In the extreme we have an ionic. This is a polar covalent bond. This property is roughly described as electronegativity If two atoms of differing electronegativity form a bond the electrons spend more time on the more electronegative atom.
Polar bonding with an. To determine whether an ionic polar covalent or nonpolar covalent bond is formed by two atoms the electronegativity difference ΔEN of the two atoms is computed. There are two types of covalent bonding.
Finally if the bond is between5 and 2 is a polar covalent bond. Melting point is higher than Nonpolar but lower than ionic. Very low melting and boiling points.
NaNO3 then is ionic while NH3 is covalent. 1 Polar versus Nonpolar Covalent Bonds. A The electrons in the covalent bond are equally shared by both hydrogen atoms.
Dont conduct electricity as a solid and are extremely polar. Types of Covalent Bonds.

Ch 5 6 Bonding Formulas And Naming Notes Ppt Video Online Download
Ionic Covalent Bonds Venn Diagram Amashusho Images
Catalyst October 64 2012 Way Back Wednesday 1
Electronegativity And Polar Covalent Bonding Dummies

Review For Unit Test 2 Chemical Bonding

Chemical Bonding Chemical Bonds Why Are We Studying Bonds Analogy Elements Periodic Tableletters Bonds Compoundswords Reactionssentences Putting Ppt Download
6 1 Electronegativity And Polarity Chemistry Libretexts

Parlak Sadaka Munching Polar Nonpolar Ionic Career Peaks Com

Invoice Processed By Ocr Software Download Scientific Diagram

8 3 Covalent Bonding Chemistry Libretexts

Unit 2 Chemical Bonding Covalent 2 4 What Are Polar And Nonpolar Bonds Aim How Do We Determine If A Bond Is Polar Do Now A Strontium Atom Differs Ppt Download

Covalent Bonds The Nice Bonds That Share Ppt Video Online Download

Parlak Sadaka Munching Polar Nonpolar Ionic Career Peaks Com
Bond Polarity Chemistry For Non Majors
Compare Ionic And Covalent Compounds

6 4 Polarity Of Molecules Introductory Chemistry
Chemystery Electronegativity And Polarity
Posting Komentar untuk "Ionic Polar Covalent Nonpolar Covalent Chart"