General Characteristics Of Noble Gases
The group of noble gases is one of the 18 groups in which the periodic table is divided. Thus the elements are known as inert gases.

Iit Jee Physical And Chemical Properties Of Noble Gases In Telugu Offered By Unacademy
Their low reactivity is because they have a complete set of electrons in their outer valence.

General characteristics of noble gases. Helium neon argon krypton xenon radon and oganesson. All of the gaseous elements other than the monatomic noble gases are molecules. Noble gas any of the seven chemical elements that make up Group 18 VIIIa of the periodic table.
Group 18 elements are gases and chemically unreactive which means they dont form many compounds. From Noble gas - Wikipedia. Noble gases are the least reactive chemical elements.
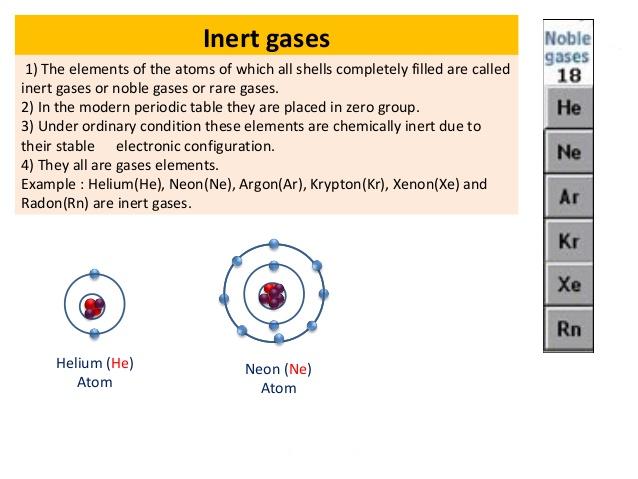
The noble gases historically also the inert gases. Under standard conditions they are all odorless colorless monatomi. On the other hand helium has 1s 2 electronic configuration.
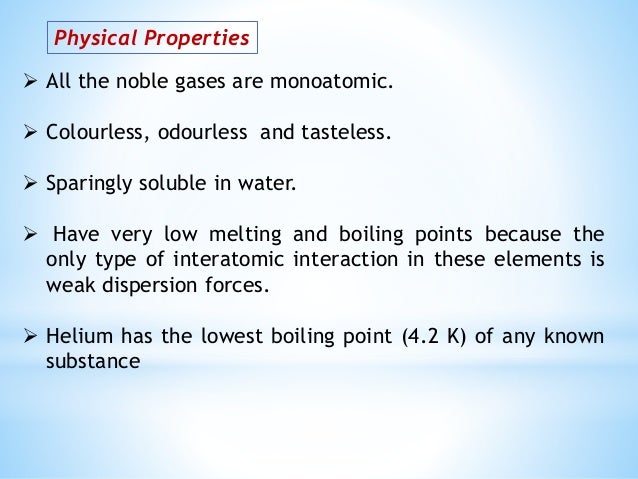
The noble gases and mercury occur as monatomic species whereas all other gases and bromine are diatomic molecules. The noble gases are colourless odourless tasteless nonflammable gases. 1- Noble gases do not usually interact with other elements.
There are seven noble gas elements. This chemical series contains helium neon argon krypton xenon and. 1 Characteristics of noble gases are odorless non-flammable colorless and monoatomic gas with low chemical reactivity.
Sometimes referred to as aerogens make up a class of chemical elements with similar properties. Other characteristics of the noble gases are that they all conduct electricity fluoresce are odorless and colorless and are used in many conditions when a stable element is needed to maintain a safe and constant environment. Group 18 elements are known as noble or inert gases.
Given this lack of reactivity their chemistry is quite limited and they tend to only react with the MOST reactive elements ie. Subscribe my channel for more videos of chemistry. The noble gases are group 18 on the periodic table which is the column of elements on the right side of the table.
This chemical series contains helium neon argon krypton xenon and radon. All the elements of group 18 ie. They are nearly inert because the atoms have.
It is composed of six elements helium neon argon krypton. Well the Noble Gases are all colourless room temperature gasesand by virtue of their closed shell electronic configuration they are i difficult to oxidize and ii difficult to reduce. In general noble gases are colorless odorless nonflammable and have a low reactivity.
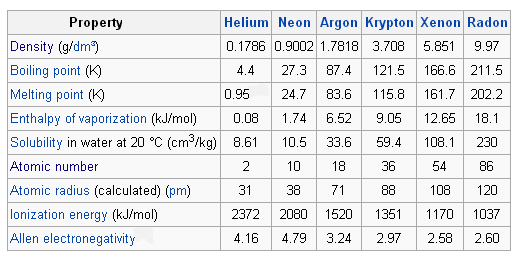
W ithin the same group 1 15 16 and 17 the lightest elements are gases. Except helium the atoms of all noble gases have eight electrons in the valence shell. The general characteristics of noble gases are discussed below.
General Characteristics of Group 18 Elements 1 Electronic Configurations. Maybe youll get different insights into general characteristics of noble gases here. Echemi shares different kinds of information about general characteristics of noble gases.
They traditionally have been labeled Group 0 in the periodic. 3 All noble gases are insoluble in water. Other characteristics of the noble gases are that they all conduct electricity fluoresce are odorless and colorless and are used in many conditions when a stable element is needed to maintain a safe and constant environment.
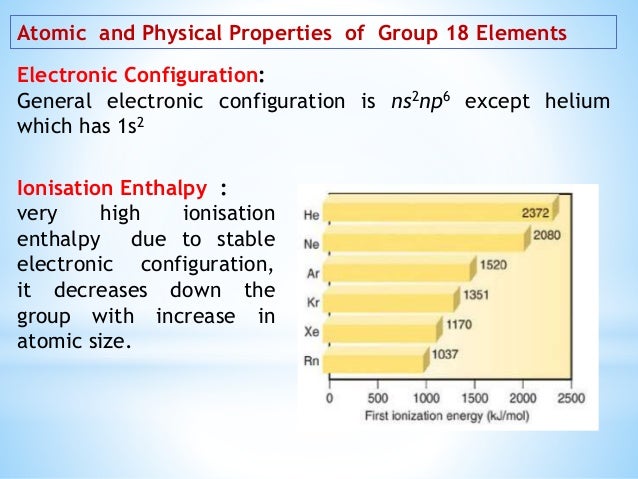
The general electronic configuration of noble gases except He may be expressed as ns 2 np 6. Like the other group elements noble gas elements also exhibit trends in their physical and chemical properties. The noble gases are also referred to as Group 8A Group 18 Group VIIIA and even Group 0.
Characteristics of noble gases. Sometimes referred to as aerogens make up a group of chemical elements with similar properties. The elements are helium He neon Ne argon Ar krypton Kr xenon Xe radon Rn and oganesson Og.
The group of Noble gases Is one of 18 groups in which the periodic table is divided. They are called inert because they do not take part in any chemical reaction and so we can say that they are chemically inert. 2 All the noble gases conduct electricity and fluorescence which can be needed in many conditions to maintain a constant and safe environment.
Helium He Neon Ne Argon Ar Krypton Kr Xenon Xe Radon Rn Organesson Og. Between the Characteristics of noble gases Most important they are gaseous elements do not interact with other elements have a full valence layer are rare in nature their level of presence on the planet Earth is low and create fluorescence. General Characteristics Of Noble Gases.
Among the characteristics of the most important noble gases are that they are gaseous elements do not interact with other elements have a full valence shell are rare in nature their level of presence on planet Earth is low and create fluorescence.

Ppt Metals Nonmetals Metalloids Noble Gases Powerpoint Presentation Id 6700407
The Noble Gases Group 18 Introduction To Chemistry
Noble Gases Group 18 Elements He Ne Ar

General Properties Of Group 18 Elements Definition Examples Diagrams

Group 18 Inert Gases Elements Occurrence Preparation Properties Structure Uses
Amazing Facts About Noble Gases

Amazing Facts About Noble Gases
Why Are Called Noble Gases What Are The Properties Of Noble Gases
The Properties Of The Noble Inert Gases Science Online

Noble Gases The Gases In Group 18 Properties Of Matter Chemistry Fuseschool Youtube

Group 18 Inert Gases Elements Occurrence Preparation Properties Structure Uses

Group 18 Elements Noble Gases Group 18 Elements

Group 18 Elements Noble Gases Ppt Video Online Download

Group 0 18 Noble Gases Physical Properties Uses Helium Neon Argon Krpton Xenon Radon Melting Points Boiling Points Atomic Radii Density Inertness Explained Gcse Chemistry Ks4 Science Igcse O Level Revision Notes
Amazing Facts About Noble Gases


Posting Komentar untuk "General Characteristics Of Noble Gases"