Do Enzymes Decrease Activation Energy
Reducing activation energysure but HOW does an enzyme catalysis reduce the energy barrierΔG. This is one of the most critical aspects of how enzymatic reactions work.
1 18 Enzymes And Allosteric Regulation Biology Libretexts
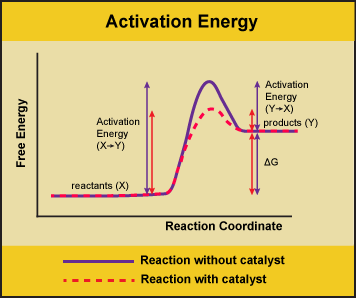
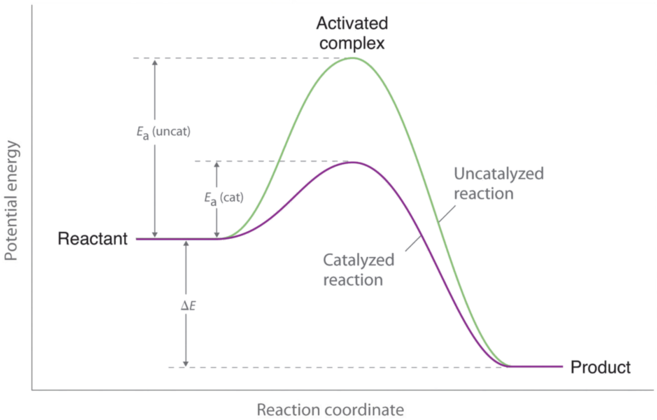
The enzyme-substrate complex can lower the activation energy by contorting substrate molecules in such a way as to facilitate bond-breaking helping to reach the transition state.
Do enzymes decrease activation energy. The enzyme and enzyme-substrate interaction decrease the react enzyme-substrate reactions activation energy and reaction rate. You may have previously been most familiar with the lock and key model of enzyme action. Enzymes accelerate reactions by decreasing ΔG the free energy of activation.
Rather they provide an alternative set of reactions with lower activation energies. Enzymes lower activation energy through various means including positioning substrates together in the proper orientation applying torque on the substrates providing the proper charge or pH microenvironment and adding or removing functional groups on the substrates. 7 Does a catalyst change the equilibrium constant.
Finally some enzymes lower activation energies by taking part in the chemical reaction themselves. Enzymes can lower the activation energy of a chemical reaction in three ways. How enzymes decrease the activation energy for a reaction.
Catalytic Mechanisms HOW do enzymes do their job. So using R for reactants C for cata. Catalysts of which enzymes are a subset do not actually affect the activation energy of the original reaction of interest.
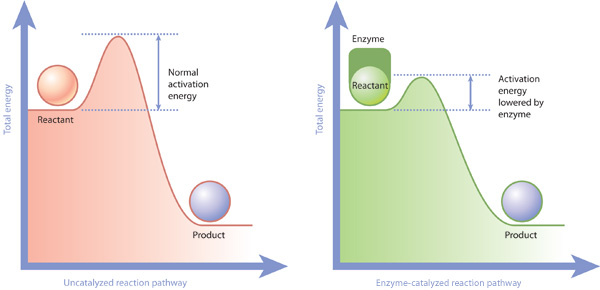
One of the ways the activation energy is lowered is having the enzyme bind two of the substrate molecules and orient them in a precise manner to encourage a reaction. The subsequent drop in energy is the energy released by the reaction. Enzymes Decrease the Activation Energy.
The enzyme does not change the equilibria. Enzymes accomplish this by lowering activation energy which is the energy required for a chemical reaction to proceed. Reaction rate By signing up youll get thousands of.
The enzyme-substrate complex can also lower activation energy by bending substrate molecules in a way that facilitates bond-breaking helping to reach the transition state. 3 How do enzymes lower the activation energy. 4 How do catalysts decrease the activation energy.
Its the amino acids. Stabilization of reactive intermediates Enzyme-catalyzed reactions generate reactive intermediates such as carbanions carbocations and radicals that. Finally enzymes can also lower activation energies by taking part in the chemical reaction itself.
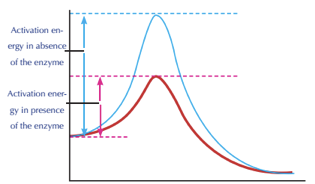
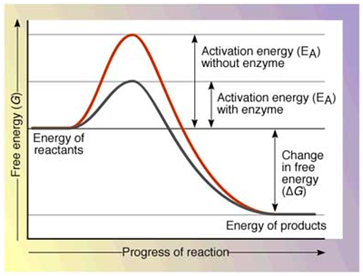
The the initial rise in energy seen in the graph left is the energy input needed before the reaction will occur activation energy. 6 Do catalysts affect activation energy of reverse reaction. This lowers the energy of the transition state and decreases the activation energy.
5 What happens when activation energy is lowered. The rate of chemical reactions can be altered by changing pH temperature andor the substrate concentration. 8 What happens as the activation energy increases the pressure of the system decreases.
On the left is a reaction that is not catalyzed by an enzyme red and on the right is one that is green. Enzymes are used in a chemical reaction to increase the reaction rate or speed and helps in the improvement of production or yield. Enzymes lower the barriers that normally prevent chemical reactions from occurring by decreasing the required activation energy.
The enzyme may reduce the reaction entropy ΔS by bringing substrates together in the correct orientation to react. The enzyme may create a charge distribution opposite to that of the transition state. Enzymes lower the activation energy necessary to transform a reactant into a product.
It is observed that enzymes are able to reduce the required. 1 question What effort do enzymes have on the activation energy of exergonic and endergonic reactions activation energy of exergonic reactions. Optimal pH increases enzyme.
Enzymes are biological catalysts for lowering activation energy - the speed up the rate of reactions and all allow biological reactions involved in metabolic processes to take place at body temperature 37C. One approach to understanding how enzymes achieve this facilitation is to assume that the transition state S and the substrate S are in equilibrium.
14 7 Catalysis Chemistry Libretexts

Aqa Gcse Chemistry Paper 2 The Rate And Extent Of Chemical Change Complete Revision Summary Notes Gcse Chemistry Chemistry Paper Chemical Changes
Factors Affecting Enzyme Activity A Level Biology Revision Notes
Enzymes Allow Activation Energies To Be Lowered Learn Science At Scitable

Biomolecules Mechanism Of Action Of Enzymes Class Eleven Biology
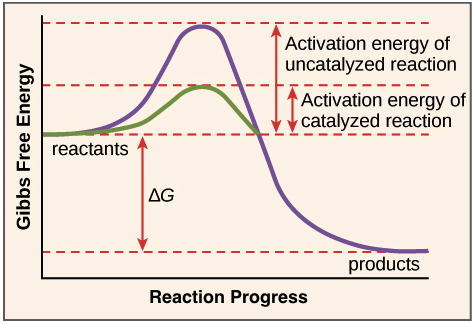
Enzymes And The Active Site Article Khan Academy

Pin By Peter Gram Jensen On Biology Competitive Inhibition Biochemistry Enzyme Inhibitor
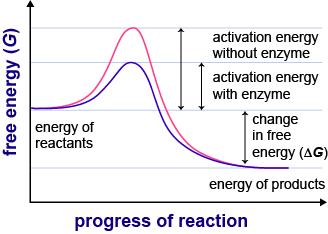
Energy Flow And Enzymes Enzymes In Detail Shmoop
Https Dls Ym Edu Tw Course Hb Doc Lecture4 11 2011 Enzymes Pdf
How To Describe The Difference Between Activation Energy And Enzymes Quora

Structural Biochemistry Enzyme Transition State Wikibooks Open Books For An Open World
Biochemistry Activation Energy Enzymes Pathwayz

Enzymes Biology For Non Majors I
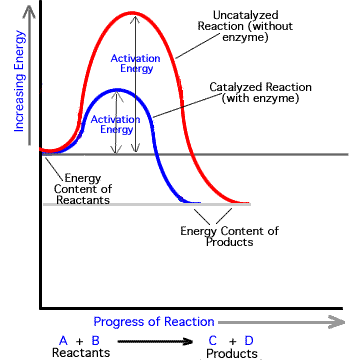
How Do Enzymes Speed Up The Chemical Reactions Use Activation Energy In Your Answer Socratic
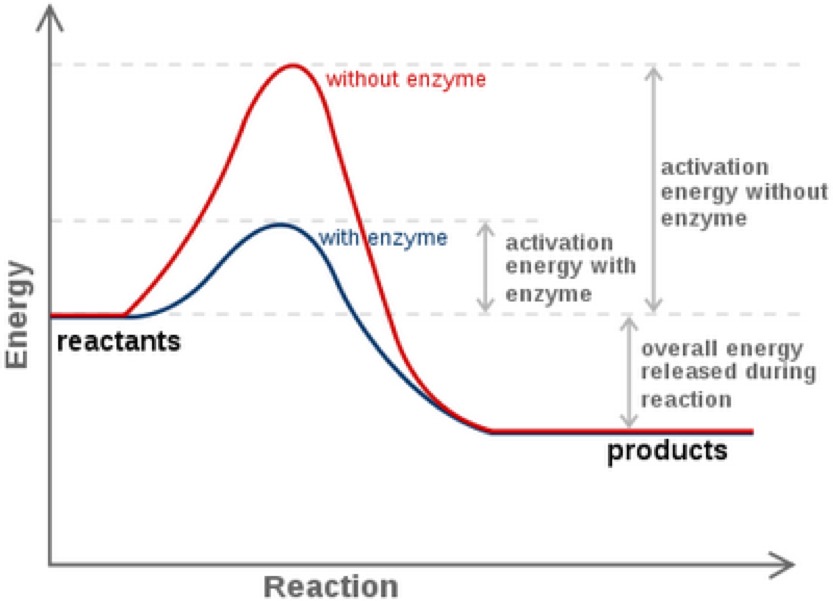
.png)
Posting Komentar untuk "Do Enzymes Decrease Activation Energy"