Most Electronegative Element List
List of Electronegativity Values of the Elements 2 This entry was posted on May 9 2015 by Todd Helmenstine updated on May 2 2021 Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The element names in 10 different languages.

What Is Electronegativity Chart List Of Electronegativity Pdf Periodic Table
Most Electronegative Element.
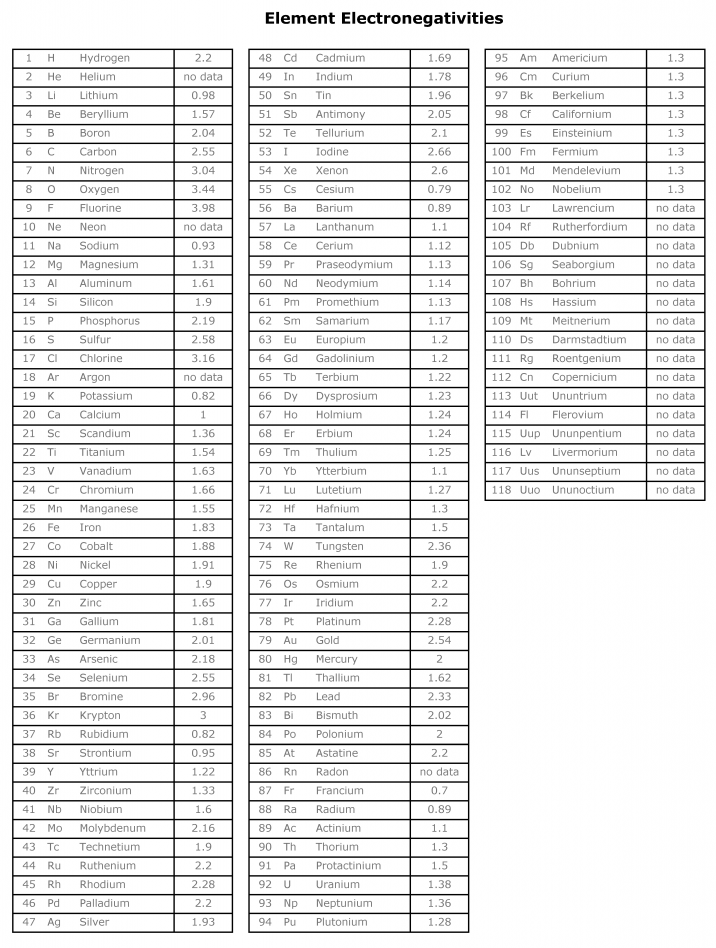
Most electronegative element list. Over 3600 nuclides isotopes. Fluorine the most electronegative element has arbitrarily been given a value of 40. 118 rows Chemical elements listed by electronegativity The elements of the periodic table sorted by.
Fluorine is followed by Oxygen Chlorine and Nitrogen is the most electronegative elements list. Electronegativity is a concept developed by Linus Pauling a rather famous chemist who won the Nobel Prize twice once for chemistry once for peace. Electronegativity measures an elements ability to form chemical bonds.
Fluorine is the highest electronegative element. The Periodic Table contains a lot more information than merely the names of each of the chemical elements. However Mulliken and using different calculations Pauling added some nuanc.
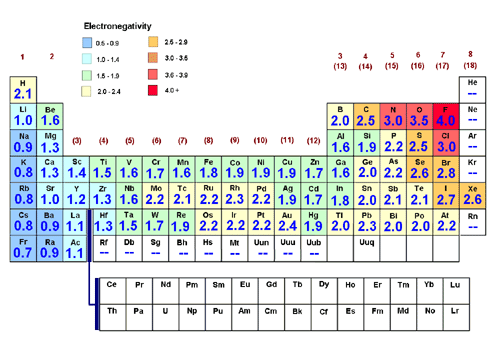
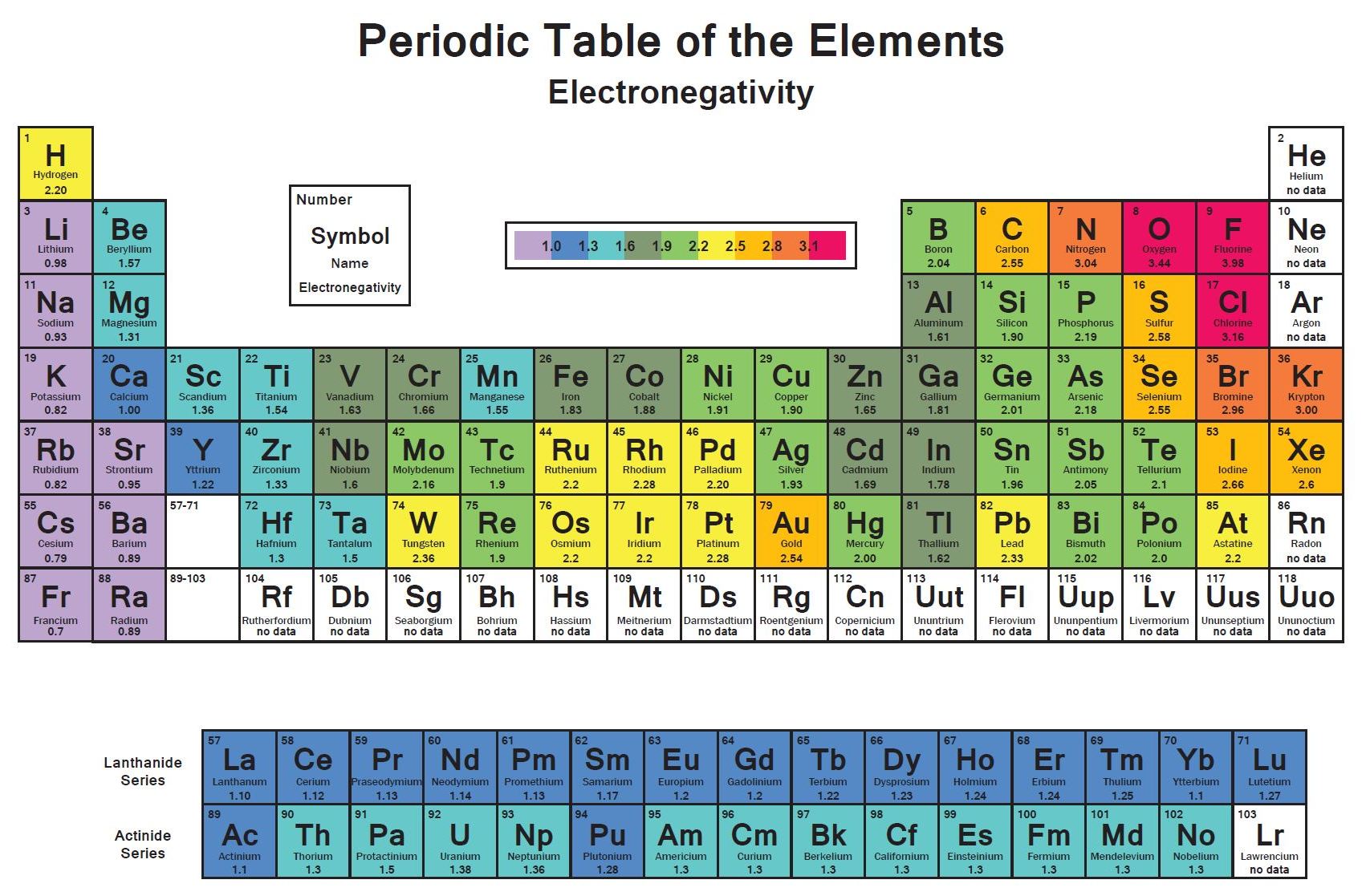
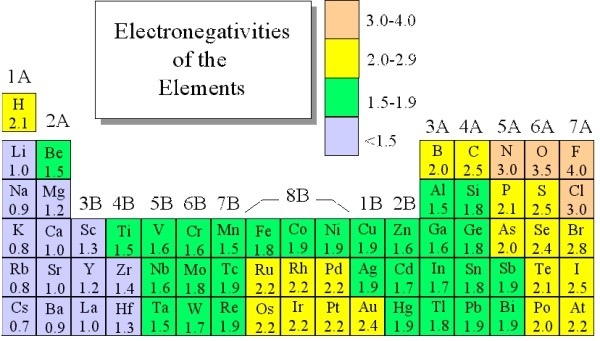
Fluorine the most electronegative element is assigned a value of 40 and values range down to cesium and francium which are the least electronegative at 07. Every other elements electronegativity has been scaled accordingly. Heres a colour coded periodic table just of electronegativity values.
The idea is to distill all the knowledge of chemistry into a single number a scale that ranges from 0 to 4. Over 4400 nuclide decay modes. The electronegativity chart describes how atoms can attract a pair of electrons to itself by looking at the periodic table you can identify and determine electronegativity values of elements from 0 to 4.
Electronegativity on the Periodic Table. The Pauling scale is the most commonly used. Electronegativity is basically how much elements want electrons.
This handy dandy chart shows rough trends and is sufficient for entry level courses Just ignore the noble gas column. Why Fluorine Is the Most Electronegative Element. You can print the.
In inorganic chemistry it is easy to estimate a single value of electronegativity to be true for most normal circumstances. That should answer your question plus any more you have relating to electronegativities. Theyre happy as they are thank you very much.
So we have fluorine oxygen nitrogen chlorine bromine iodine sulfur carbon and hydrogen. The base value of hydrogen was later increased by 010 and caesiums electronegativity was later refined to 079. Each element has an electronegativity value.
And if you look at this list you might recall some of. Electronegativity rises from bottom to top in groups and jumps from left to right across periods. This is the most electronegative elements on the periodic table starting with the most electronegative on the top and decreasing in electronegativity as we work down.
In addition chemistry and technical terms are linked to their definitions in the sites chemistry and environmental dictionary. As we already mentioned above the most electronegative element is fluorine followed by oxygen. Electronegativities of the elements data page The electronegativity of francium was chosen by Pauling as 07 close to that of caesium also assessed 07 at that point.
When you are looking at an electronegativity chart and you want to establish some patterns the best thing you can do is to think about fluorine since it is the most electronegative element in the Periodic Table. Other highly electronegative elements are oxygen and chlorine. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H.
Up to 40 properties chemical. Fluorine has an electronegativity of 398 on the Pauling Electronegativity Scale and a valence of 1A fluorine atom needs one electron to fill its outer electron shell and achieve stability which is why free fluorine exists as the F-ion. A simple way to think about it is that the closer an element is to Fluorine the higher its electronegativity is.
The Patterns Of Electronegativity In The Periodic Table. However no refinements have been. 96 rows To list the elements order by electronegativity click on the table headers.
This site offers comprehensive information for each element including. Again thats FONCLBRISCH. Its monatomic form H is the most abundant chemical substance in the Universe constituting roughly 75 of all baryonic mass.
Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Who when. Elements with electronegativities of 25 or more are all nonmetals in the top right-hand comer of the periodic table.
Helium is the most electronegative element followed by Fluorine. Who are the 2 most electronegative elements on the periodic table. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table.
Here in the given options Oxygen is the most electronegative element. By signing up youll get thousands of step-by-step solutions to your. Fluorine is the most electronegative element.
The most electronegative element is Fluorine with a score of 40 the highest possible Across from Fluorine we also have N and O with high electronegativities. The red end of the spectrum indicates the highest electronegativities.

Difference Between Electropositive And Electronegative Compare The Difference Between Similar Terms

Electronegativity Chart Click To Download Free Pdf

Electronegativity Table Easy Hard Science
Is Carbon An Electronegative Element Quora
Which Of The Following Is The Most Electronegative Carbon Lead Tin Or Silicon Quora
Illustrated Glossary Of Organic Chemistry Electropositive
Select The Most Electronegative Element From The List Chegg Com
What Trend In Electronegativity Do You See As You Go Across A Period Row On The Periodic Table Socratic
Why Are The Electronegativity Values Of Noble Gases Zero Socratic
Electronegativity And Electronegativity Chart In Pdf Chemistry Com Pk
Electronegativity Chart Of Elements List Of Electronegativity

Electronegativity Definition Oxidation Number Non Polar Bond

Electronegativity Basic Introduction Periodic Trends Which Element Is More Electronegative Youtube
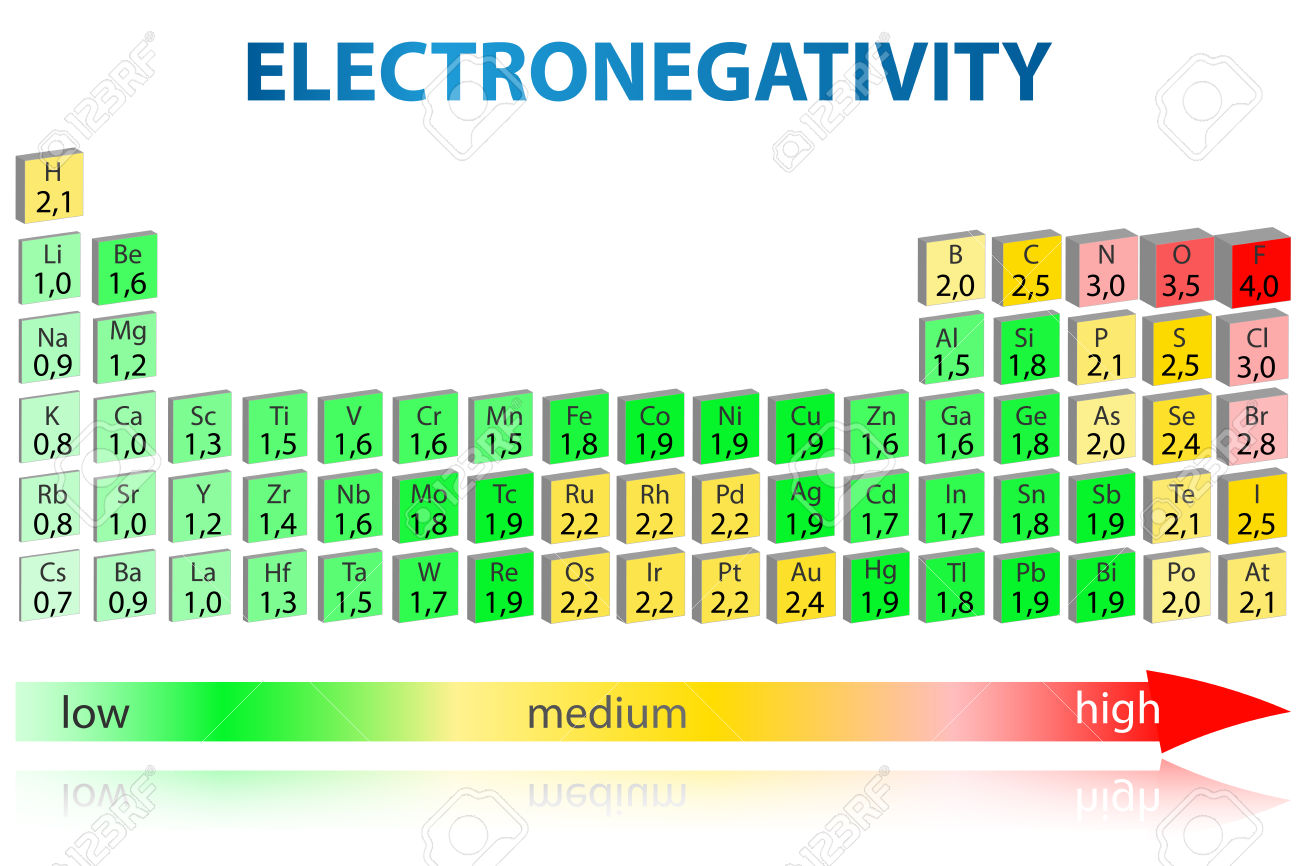
What Elements Has The Highest Electronegativity
What Are Some Of The Most Electronegative Elements In Nature Quora

Electronegativity And Electronegativity Chart In Pdf Chemistry Com Pk

Posting Komentar untuk "Most Electronegative Element List"