Ph Of Natural Rain
I try to collect after it has rained for about 10 minutes or I just make it easy and throw a old sheet over the can to keep fine debris out. Rain -water normally has a pH of 56 due to the formation of carbon dioxide present in the atmosphere.
Acid rain is rain with a pH of 5 and below.
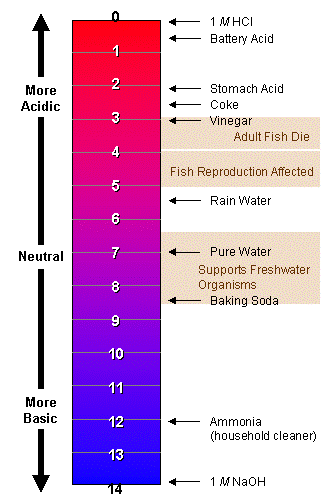
Ph of natural rain. The EPA has some great ideas for acid rain experiments so today were sharing one that measures the pH of natural water using our pH test strips. When the pH of the rain drops below 56 it becomes acidic. The scale has values ranging from zero the most acidic to 14 the most basic.
Depending on region season and presence of pollutants the pH of rain may drop to as low as 20 the acidity of vinegar. Unpolluted rain in the atmosphere has an acidic pH if unaffected by pollutants such as sulphur dioxide and oxides of nitrogen and the acidic content of natural rainwater is largely caused by the. The pH of rainwater is normally from 6 - 65.
The pH scale measures how acidic an object is. Acid Rain and the pH Scale. The National Atmospheric Deposition Program has developed maps showing pH patterns such as the one below showing the spatial pattern of the pH of precipitation at field sites for 2002.
However considering all chemical compounds in the environment leads to modern scientists deducing that the pH is in the region of 5 to 7. EPRI also contends that pH 56 may or may not be a valid reference point. It is often assumed14 that the pH of natural rainwater is controlled by the dissociation of dissolved CO2 has a value of 56 and that decreases below this are due to the addition of acidic.
However natural unpolluted rainwater actually has a pH of about 56 acidicRecall from Experiment 1 that pH is a measure of the hydrogen ion H concentrationThe acidity of rainwater comes from the natural presence of three substances CO 2 NO and SO 2 found in the troposphere the lowest layer of the atmosphere. Natural variations and human pollutants may cause rain to be more acidic. Natural soil pH reflects the combined effects ofsoil-forming factors parent material time relief or topography climate and organisms.
See also the Report Sheet and Questions pages for this laboratory. Most grasses and legumes prefer soils with a pH of 45-70 so the slight acidity of rain can benefit carbonate soils ²³. Objects that are not very acidic are called basic.
Even without man-made influences there are natural sources of sulfur oxides nitrogen oxides and other species important to determining the precipitation acidity at any given time. Pure water has a pH of 70 neutral. Complete and turn in the Pre-Laboratory Assignment from.
Natural rain water is slightly acidic pH 56 since there is carbon dioxide in the atmosphere that dissolves to make carbonic acid. As you can see from the pH scale above pure water has a pH value of 7. Temperature and rainfall control leachingintensity and.
Acid Rain and the pH of Natural Waters. Actually the acidity of rain water is increased due to the large quantity of SO2 and oxides of nitrogen present in the air. Pure water has a pH of 70 neutral.
What would be the pH of rainwater if. Read the TECH 337 Laboratory Techniques. The pH of pure water is 7.
The traditional measure of the natural pH of rainwater is around 57. However there are always impurities in rainwater because it stays in equilibrium with carbon. The pH of precipitation and water bodies vary widely across the United States.
Natural Acidity of Rainwater. However natural unpolluted rainwater actually has a pH of about 56 acidicRecall from Experiment 1 that pH is a measure of the hydrogen ion H concentrationThe acidity of rainwater comes from the natural presence of three substances CO 2 NO and SO 2 found in the troposphere the lowest layer of the atmosphere. It should not be considered the background or natural acidity of precipitation.
The acidity of the surrounding environment can also affect the pH. Natural precipitation both rain and snow has a pH near 56 due to contact with CO2 and other atmospheric influences. This value is considered neutralneither acidic or basic.
Im in VA USA my rain water phis about 68 and the TDS is a low of 4 ppm to a high of 12 ppm depending on the debris in my gutters and how much rain we have had to keep the gutters flushed out. Rain water is naturally slightly acidic witha pH of about 50. Natural and human processes determine the pH of water.
The pH of newly formed soils is determined by minerals in the soils parent material.

Ph Scale Diagram Google Search

The Queen Of Dental Hygiene Tooth Decay What S Ph Got To Do With It The Final Wrap Up For Now Natural Hair Styles Hair Diary Your Hair

Ph Balance Chart For Organic Hair Care Organic Hair Organic Hair Care Hair Care

The Ph Scale For Carpet Cleaners Teaching Chemistry Chemistry Lessons Biology Lessons

Cleaning And Ph Levels Kombucha Ph Chart Alkaline Foods

Pin On Natural Wellness Beauty

Changing Ecosystem Concerns Fishermen The Portland Press Herald Maine Sunday Telegram Teaching Chemistry Chemistry Classroom Gcse Science

Rain Palette Aims To Provide An Easy And Poetic Approach To Visualising Air Quality Through Rainwate Visualisation Data Visualization Color Palette Living Room

Tipps Und Tricks Fur Eine Gesunde Jugendliche Haut Womens Skin Care Routine Skin Care Business Skin Care Oily Skin Care

Understanding The Importance Of Ph The Skincare Diary Skin Care Pimples Skin Care Business Skin Care








Posting Komentar untuk "Ph Of Natural Rain"