What Is The Least Electronegative Element
When atoms have gained at least one electron they are known as anions. 103 rows This is especially problematic for francium which by relativistic calculations can be shown to be.

The Parts Of The Periodic Table
The element with the lowest electronegativity value is francium which has an electronegativity of 07.

What is the least electronegative element. Has the least electronegativity Francium. Thus fluorine is the most electronegative element while francium is one of the least electronegative. The least electronegative element is francium according to Paulings electronegativity scale which does not include other electronegative elements.
119 rows Electronegativity is a property which tells how well an atom can attract an electron to. The most electronegative element in fluorine. The Allen scale assigns the lowest electronegativity to cesium with a value of 0659.
And the atoms that are lost at least one electron are known as cations. While anions are negatively charged cations are positively charged. It describes the general trend across the periodic table and explains ho.
Electronegativity of Fluorine Fluorine is the most electronegative element because it has 5 electrons in its 2P shell. The least electronegative elements are cesium Cs and francium Fr with electronegativity values of 07. The scale is named after Linus Pauling an American chemist.
This value uses the Pauling scale to measure electronegativity. Francium has an electronegativity of 067 on that scale. The optimal electron configuration of the 2P orbital contains 6 electrons so since Fluorine is so close to ideal electron configuration the electrons are held very tightly to the nucleus.
See full answer below. Fluorine the most electronegative element is assigned a value of 40 and values range down to caesium and francium which are the least electronegative at 07. Its electronegativity value is 079.
The atoms that have either gained or lost one or more electrons are known as ions. The least electronegative element is francium according to Paulings electronegativity scale which does not include other electronegative elements Why does electronegativity happenWhich element has the highest Electropositivity. The least electronegative element is francium according to Paulings electronegativity scale which does not include other electronegative elements like argon neon and helium.
The alkali metals are the most electropositive. 96 rows You can print the list of elements by hitting the print button below. The least electronegative group is VIII the inert gases.
The least electro negative group is VIII the inert gases. What is the element with the lowest electronegativity value. Why is fluorine the least electronegative element.
Alkali elements are the least Electronegative. For a given Period those have the Small nuclear charge and also the lightest shielding by other electrons only the one-electron is now in their valence shells. Become a member and.
Most and Least Electronegative Elements Cesium is the least electronegative element. The element which has the. This chemistry video tutorial provides a basic introduction into electronegativity.
Its the tendency for an atom to attract a shared electron to itself. Now go diagonally across the periodic table to the heaviest alkali metal and you will find the element that is most likely to give up an electron ie. Cesium is the most electropositive of the stable elements.

Quick Answer Which Of The Elements Below Has The Largest Electronegativity The Biggest
Which Elements Have Zero Electronegativity Quora

Periodic Trends Electronegativity Chemistry For Non Majors
Illustrated Glossary Of Organic Chemistry Electronegativity

The Parts Of The Periodic Table
What Is The Order Of Electronegativity Of A Boron Family Quora
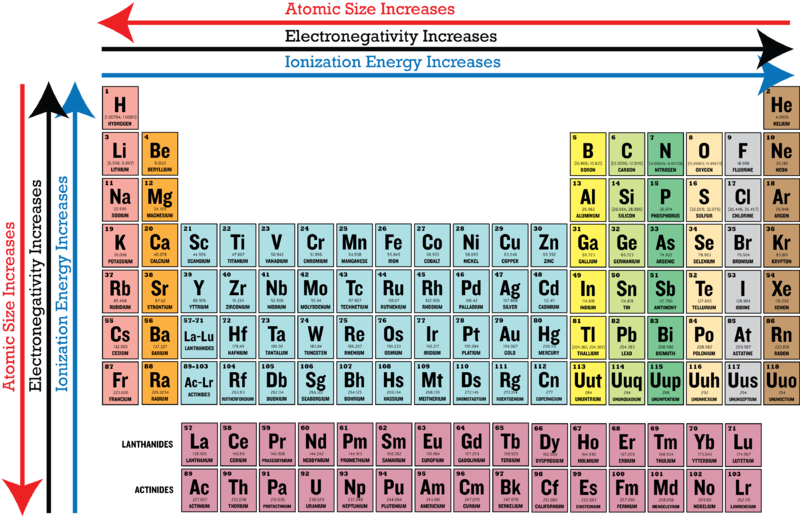
Which Of The Following Elements Has The Lowest Electronegativity Class 12 Chemistry Jee Main
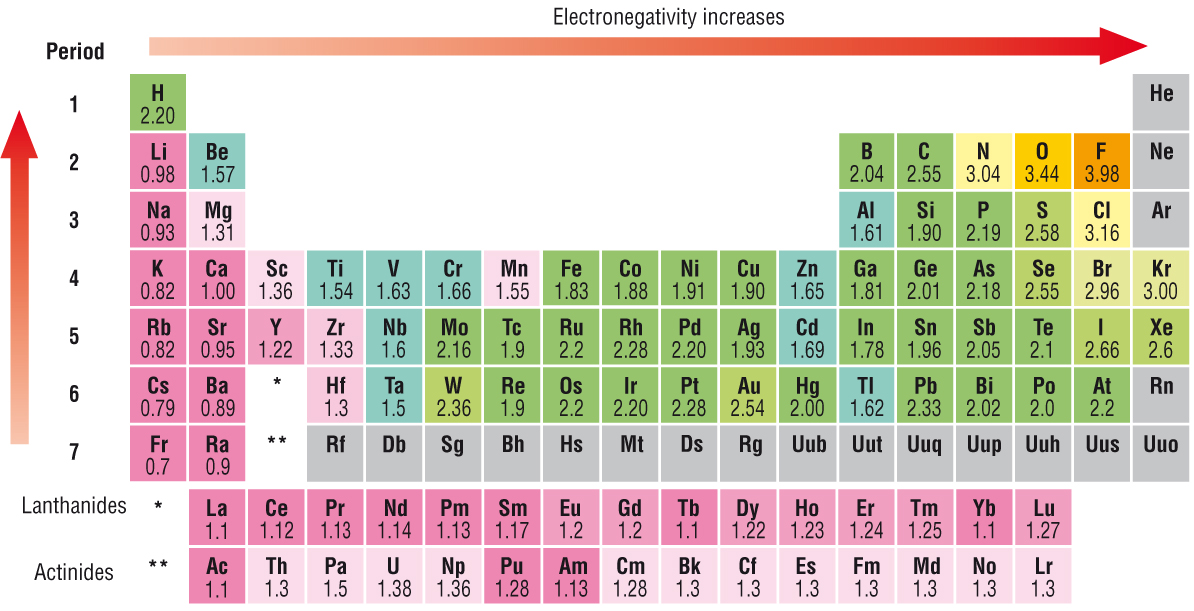
What Would Cause An Atom To Have A Low Electronegativity Value Socratic

Electronegativity Definition Oxidation Number Non Polar Bond
Why Does Helium Have No Electronegativity Quora

What Is Electronegativity Trends Chart Periodic Table Chemtalk
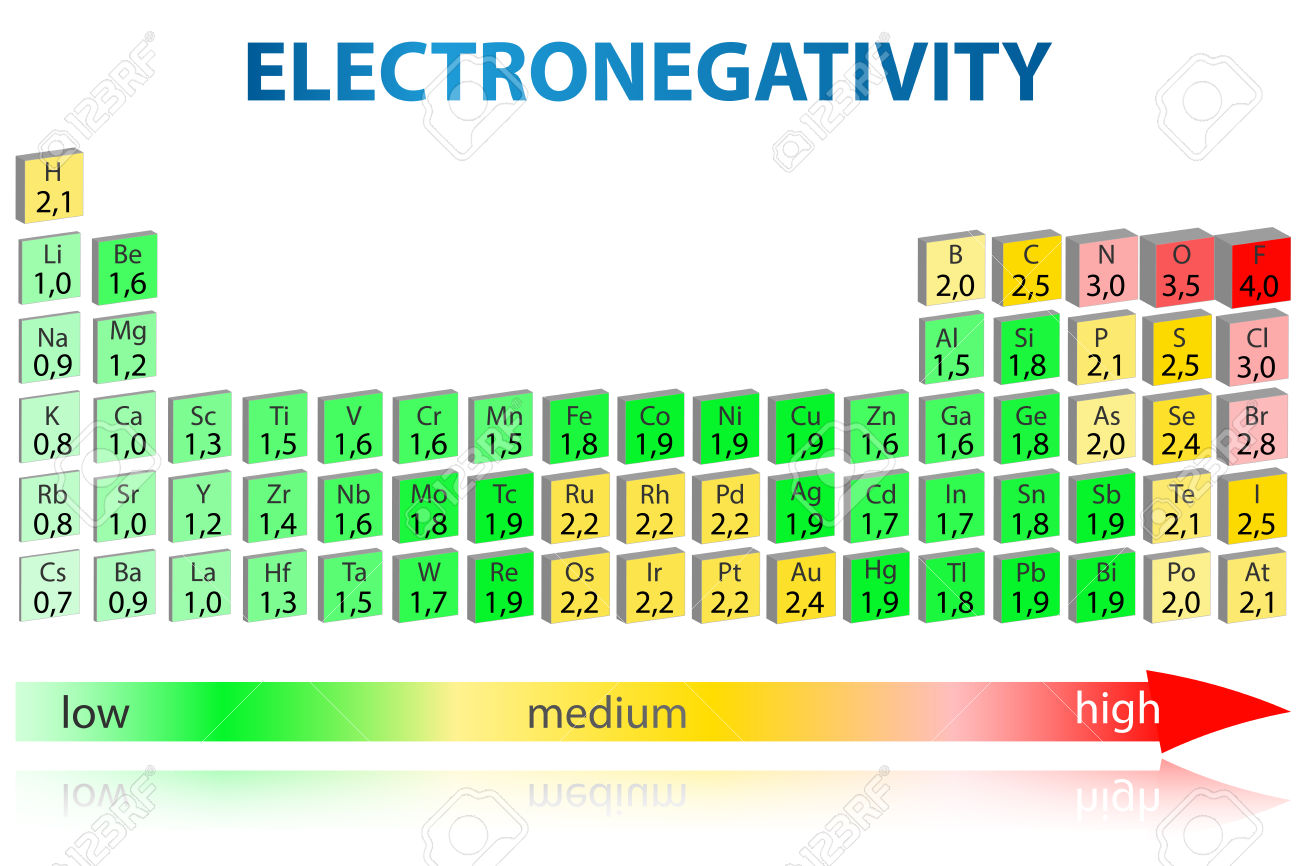
What Elements Has The Highest Electronegativity
Electronegativity Ions Atomic Size Electronegativity
Electronegativity Physical Science
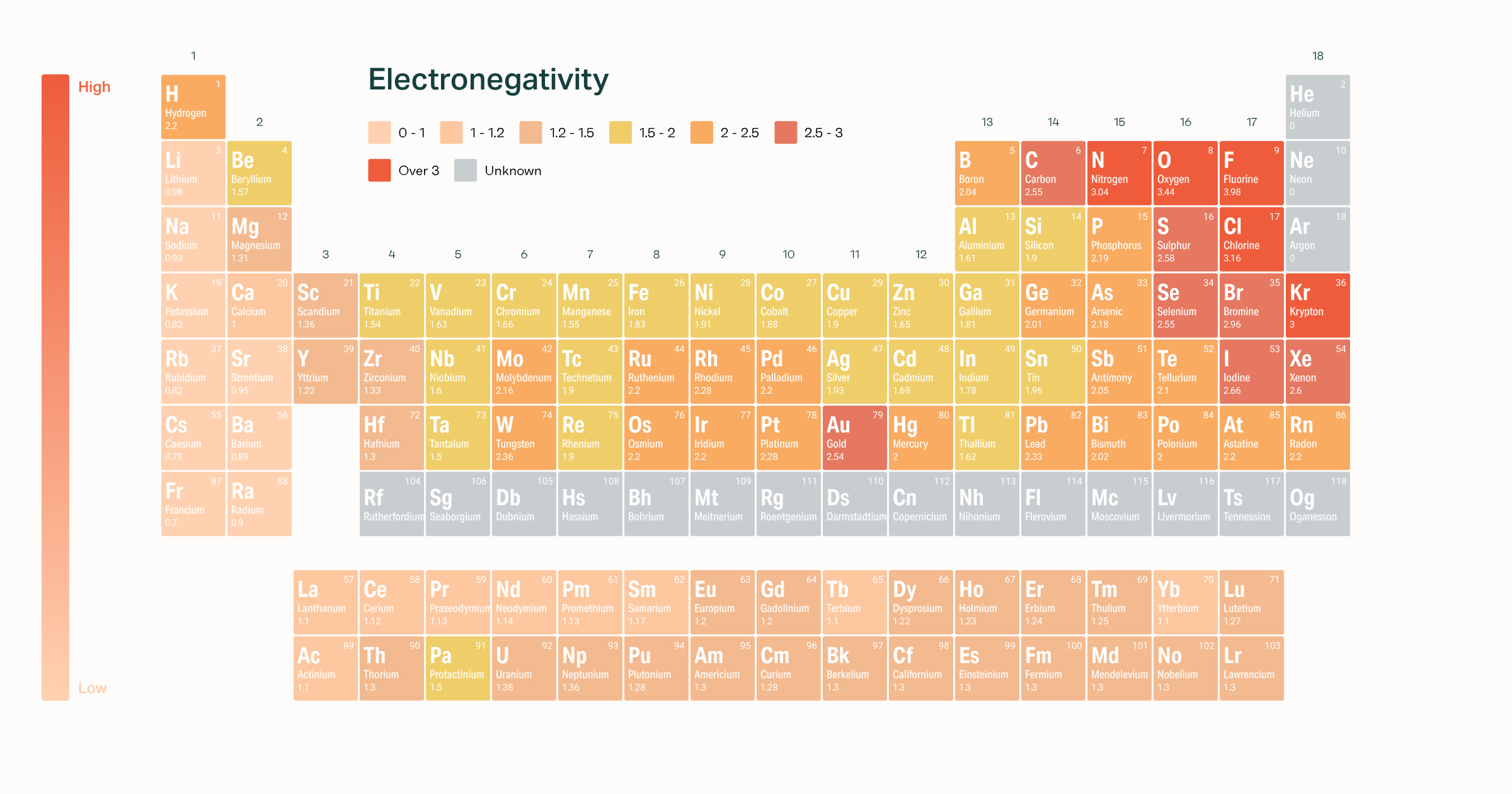
Electronegativity Of The Elements
Posting Komentar untuk "What Is The Least Electronegative Element"