How To Count Carbon Atoms In Organic Chemistry
For example the simplest alkane is CH 4 methane and the nine-carbon alkane CH. Either way it opens and reversibly dehydrates to the aldehyde R-C OH.

Primary Secondary Tertiary In Organic Chemistry
The number in the name tells you where the double bond starts.
How to count carbon atoms in organic chemistry. Now we add enough hydrogen atoms to give each carbon four bonds. The most common ring compounds contain either 5 or 6 carbons. The Wikipedia article states.
How many atoms are present in a molecule of carbon. The location of the double bond s is are indicated before the parent name as before and the location of the carbonyl group s is are indicated between the -en and -one suffixes. 0 Comments Add a Comment.
The first few are. The method used to count carbon atoms will depend on how the structure is represented. But since we have a 5 in front of the entire term well need to multiply by 5 15 5 75 O.
Get the answers you need now. The obvious answer first If the molecule is written out in Lewis structure meaning you can see every atom just count your carbons. Delores6453 21042019 Chemistry Secondary School 10 pts.
Attach two more carbon atoms to Carbon 3 using medium connectors Note. However I am feeling that a carbon containing two hydrogen cannot be considered prochiral. But counts 4 carbon atoms in the longest chain and en tells you that there is a carbon-carbon double bond.
Many organic compounds contain rings of carbon atoms or other atoms such as oxygen or nitrogen. The word roots such as meth. These compounds are also called.
Posted How Do You Count Carbon Atoms In Organic Chemistry. This can be explained by one of the important properties of carbon. Eth prop but denote the number of carbon atoms in the carbon chain.
Straight-chain alkanes take the suffix -ane and are prefixed depending on the number of carbon atoms in the chain following standard rules. The carbons are numbered starting with the carbon with the alcohol functional group. Answered How do you count carbon atoms in organic chemistry.
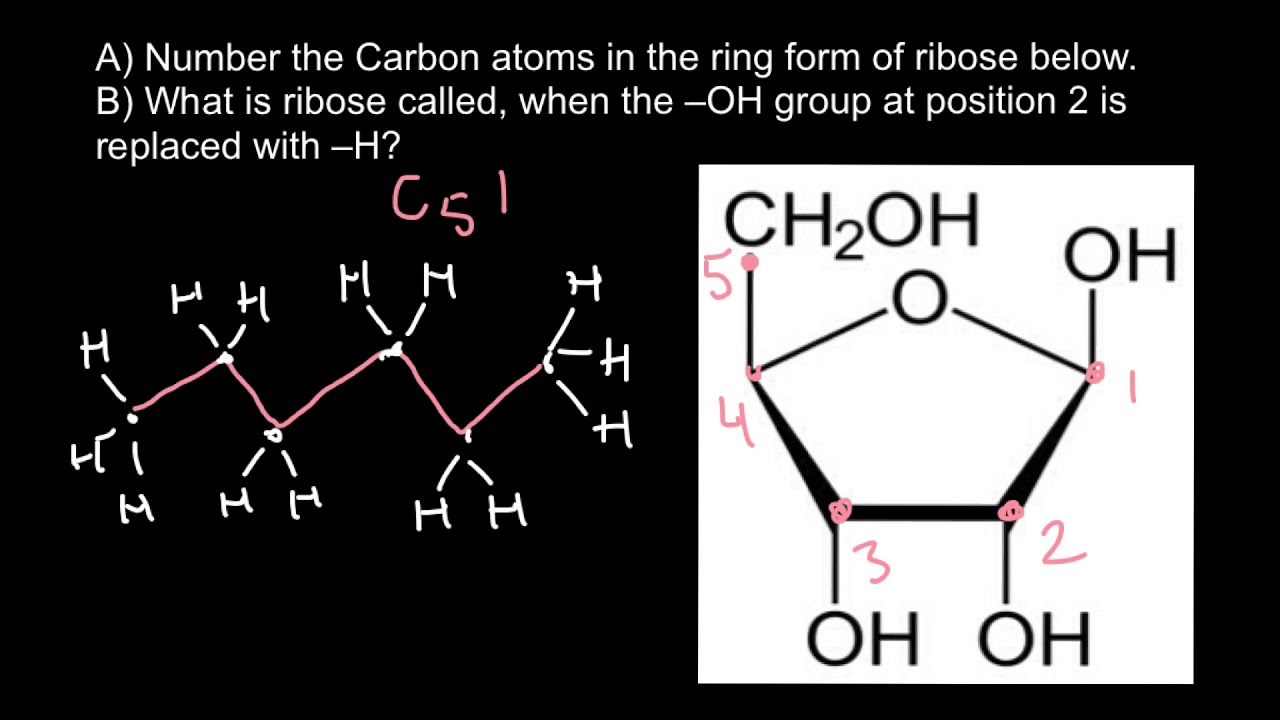
The compound butane has this structure but there is another way to put 4 carbon atoms and 10 hydrogen atoms together. The simplest ring compound contains 3 carbons as in cyclopropane. How Do You Number Carbons In Ketoses Socratic.
But H atoms are not considered substituents in organic chemistry see substituent. Double bonds count double their other end atom. Complete the structure by connecting two hydrogen atoms each to the added carbon atoms.
Secondary 2 carbon atom bonded to two other carbon atoms Tertiary 3 carbon atom bonded to three other carbon atoms Quaternary 4 carbon atom bonded to four other carbon atoms. Download for free from a curated selection of How Do You Count Carbon Atoms In Organic Chemistry Socratic for your mobile and desktop screens. See below for examples.
The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. The cyclic forms C1 also has the largest summed mass attached two oxygens plus a carbon. No number was necessary in the propene example above because the double bond has to start on one of the end carbon atoms.
April 26 2017 atoms carbon Chemistry Education Educators k-12 organic. Related Images of How Do You Count Carbon Atoms In Organic Chemistry Socratic. So if we can count O counting H atom wont break any sweat.
If two identical substituents are attached to a sp3-hybridized atom the descriptors pro-R and pro-S are used to distinguish between the two. If you prefer to process it directly it would be 4 3 1 3 5 75 O. While this sounds simple I find that students get confused on exams so I recommend using a highlighter or colored pen.
Number one atom in a chain is the end that bears the highest total mass. Comes out to the same answer just depends on how your brain works. Again the carbonyl gets priority in the numbering of the parent chain.
If carbon chain has only one one carbon atom word root is methIf number of carbon atoms in the chain is two eth is the word root used in IUPAC nomenclature in organic chemistry. After we know which elements are there in an organic compound then. Place 3 of the carbon atoms in a row and then branch the fourth one off the middle carbon atom.
Meth Eth Prop But Table - Word Roots in IUPAC Nomenclature. There are various ways to qualitatively determine which elements are present in an organic compound for eg- Lassign test for detection of nitrogenhalogenSulphur and many other. How do you count carbon atoms in organic chemistry.
Since the 3 rd Carbon has three other carbon atoms attached to it then the 3 rd Carbon is considered a Tertiary carbon. 1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023.
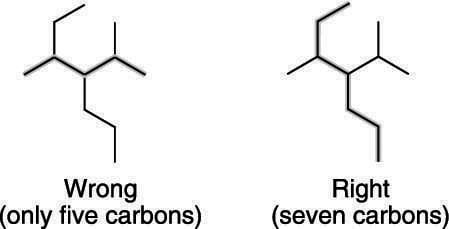
How To Find And Number The Longest Chain In A Branched Alkane Dummies

Welcome To The Oc Organic Chem Ob Mastering The Alkanes The Alkenes And The Alkynes Take Out Table P Q Now At The End Of Today You Should Be Able
Organic Nomenclature Number Carbons

Organic Chemistry The Study Of Carbon And Most Carbon Compounds Ppt Download

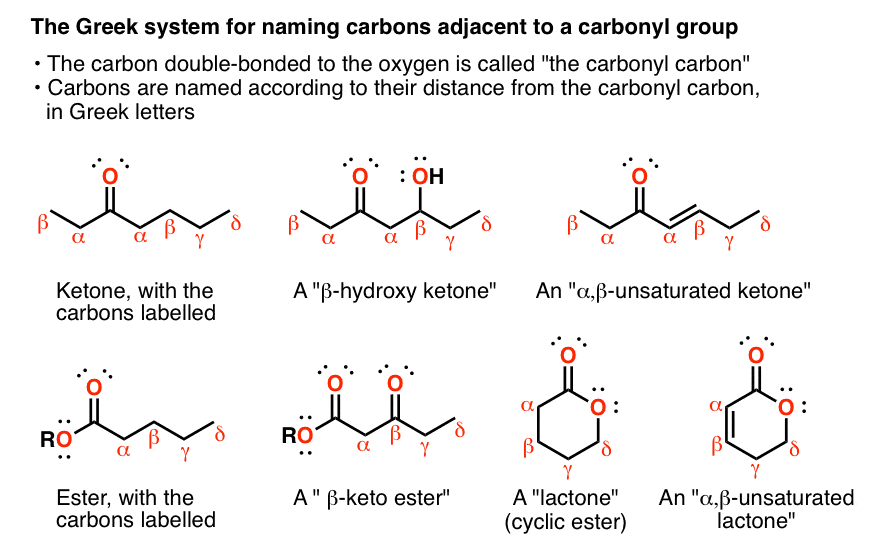
What S The Alpha Carbon In Carbonyl Compounds
How To Number Carbon Atoms Youtube
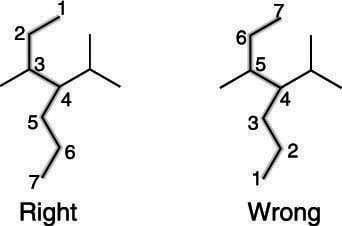
How To Find And Number The Longest Chain In A Branched Alkane Dummies
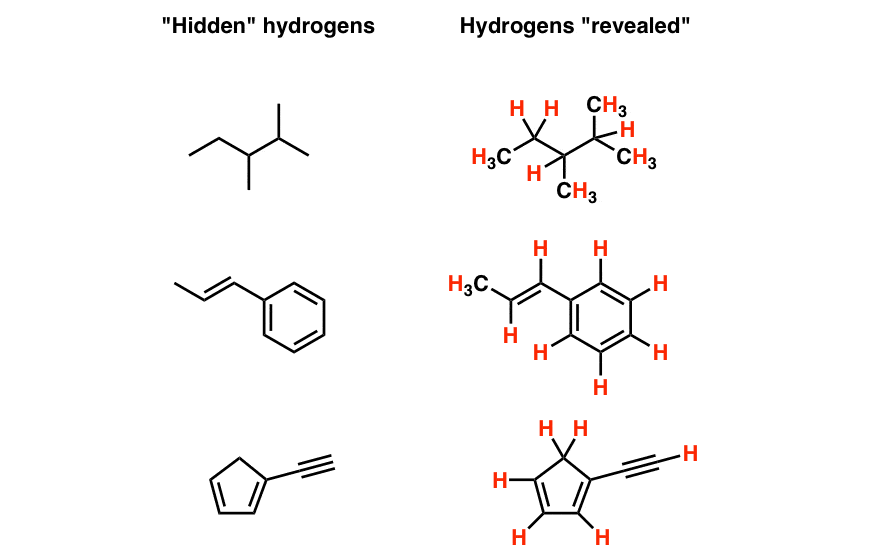
Hidden Hydrogens Hidden Lone Pairs Hidden Counterions Master Organic Chemistry

What Is The Convention Of Numbering Carbon Atoms In Organic Molecules Chemistry Stack Exchange
How To Number Carbon In Nmr Signal According To Iupac Sytem

Convention For Numbering Of Carbons Chemistry Stack Exchange

Numbering Of Carbon Atoms Youtube
Organic Nomenclature Number Carbons
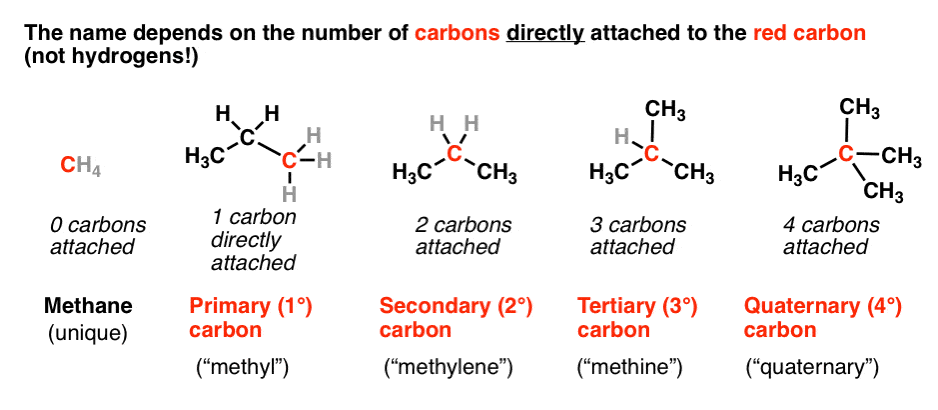
Primary Secondary Tertiary And Quaternary In Organic Chemistry
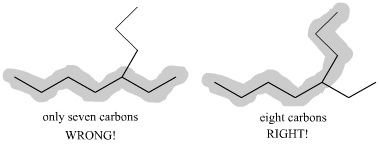
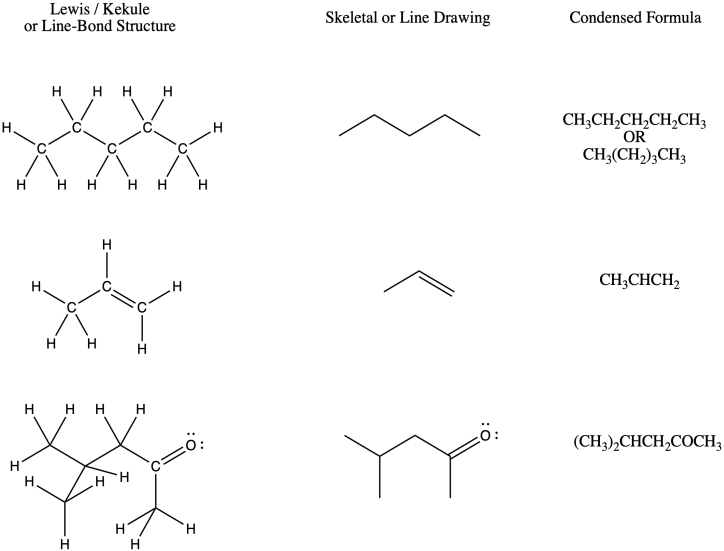

Posting Komentar untuk "How To Count Carbon Atoms In Organic Chemistry"