Jj Thomson Atomic Model Explanation
Many scientists studied the electric discharge of a cathode ray tube. Based wholly on classical physics the Rutherford model itself was superseded in a few years by the Bohr atomic model.
Thomson Atomic Model And Limitations Development Of Atomic Model
During cathode ray tube experiment a negatively charged particle was discovered by JJ.
Jj thomson atomic model explanation. It was strongly supported by Sir Joseph Thomson who had discovered the electron earlier. In this model the positive charged is uniformly distributed. He also agreed with Nagaoka that the electrons move in circular orbits outside the nucleus.
Thomsons model was correct at the time because it explained all that scientists then knew about the atom. That electrons are negatively-charged particles and that atoms have no net electric charge. Thomsons Atomic Model also called as Plum Pudding Model was the most accepted Atomic Model during the year 1904-1910 which emphasized on the inner structure of the AtomThis post will discuss what is Thomsons Atomic Model postulates of JJ.
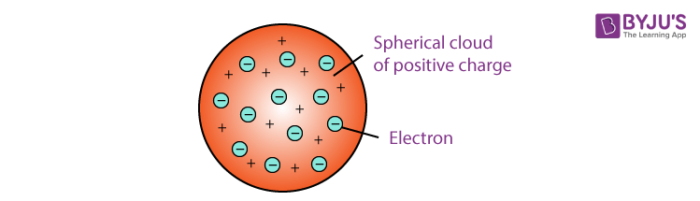
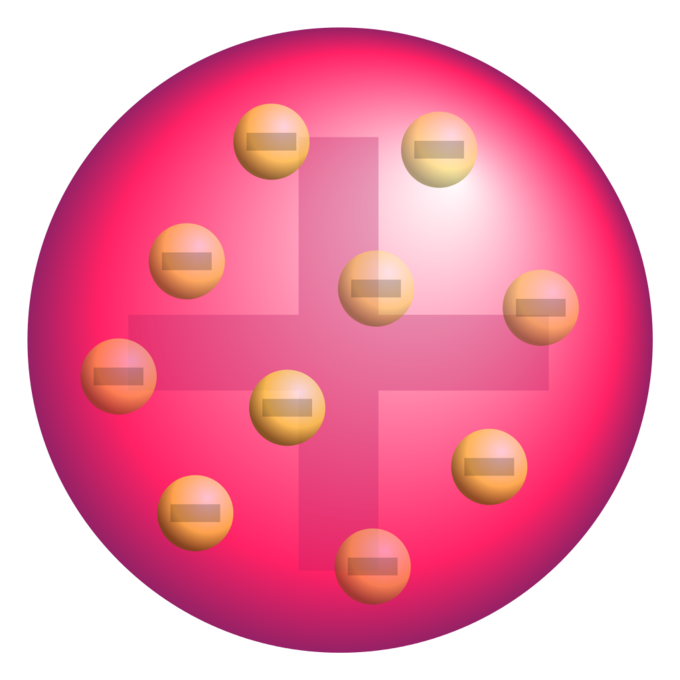
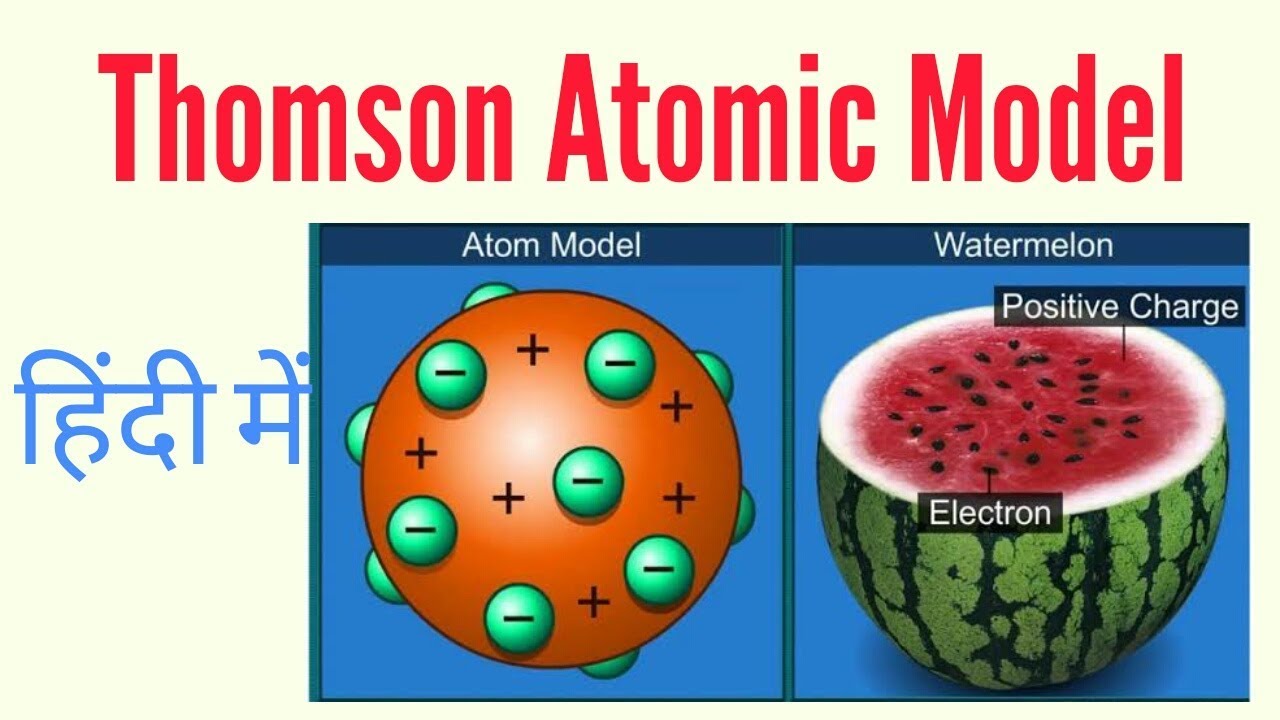
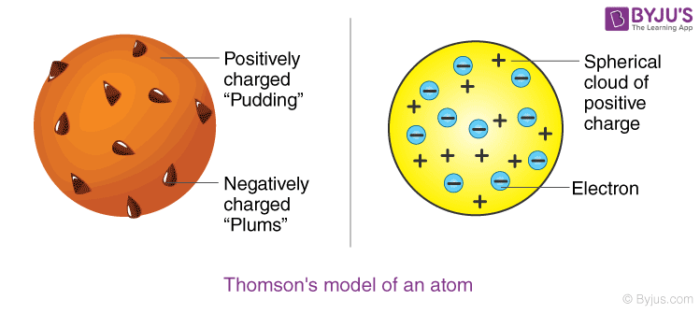
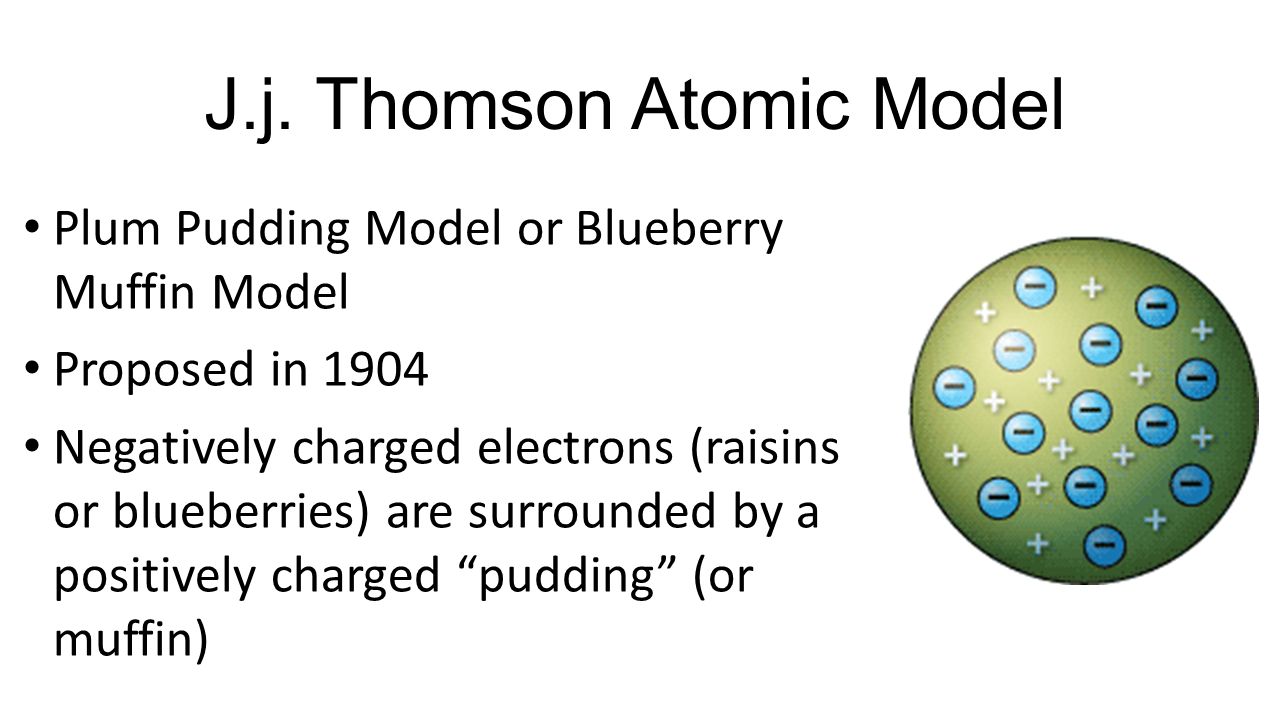
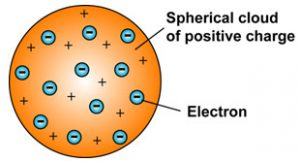
Thomson proposed the plum pudding model of the atom which had negatively-charged electrons embedded within a positively-charged soup. There were some inconsistency in his atomic theory and his atomic model. That electrons are negatively charged particles and that atoms have no net electric charge.
Thomson who is also known for the discovery of the electron. Thomson is credited with the discovery of the electron the negatively charged particle in the atom. Thomsons experiments with cathode ray tubes showed that all atoms contain tiny negatively charged subatomic particles or electrons.
Thomson Atomic Model Theory and experiments. Cathode rays are normally invisible beams of particles that can be contained within vacuum tubes for observation. First proposed by J.
It was proposed by JJ Thomson in the year 1904 just after the discovery of electrons. Thomsons Model How does Plum Pudding Model Work applications and limitations. If cathode rays are.
The distribution of positive and negative particles was not uniform. The atomic model of Thomson is the first theoretical description of the internal structure of atoms proposed around 1900 by William Thomson Lord Kelvin strongly supported by Sir Joseph John Thomson who had discovered 1897 the electron part negatively charged of each atom. Thomson soon after the discovery of the electron but before the discovery of the atomic nucleus.
Thomson Model of an atom The description of Thomsons atomic model is one of the many scientific models of the atom. It was Thomsons interpretation that was important. Atomic Model gives the explaination about the distribution of the charged particles.
Thomson in which the electrons were embedded in a positively charged atom like plums in a pudding. This model explained the description of an inner structure of the atom theoretically. The electrons are present in such a way to give the stable electrostatic arrangement.
The plum pudding model was first proposed by the English Physicist Sir Joseph John JJ. The Rutherford model supplanted the plum-pudding atomic model of English physicist Sir JJ. Here is when part 1 of John Daltons theory is disproven because it states.
The model tried to explain two properties of atoms then known. Thomson was experimenting with cathode rays. Limitations of Atomic Theory by JJ Thomson Since the atomic model constructed by JJ Thomson was one of the earliest atomic theory its understandable that his atomic theory still contains some flaws.
JJ Thomson proposed the neutrality in the atom. Thomson in 1904 soon after the discovery of the electron but before the discovery of the atomic nucleus the model tried to explain two properties of atoms then known. Rutherford showed that the atom contains a small massive positively charged nucleus.
In 1911 Rutherford showed that Thomsons model was wrong. From his cathode-ray tube experiments he realized that atoms consisted of negatively particles electrons which he called corpuscles. People Also Asked What is the atomic model of jj thomson.
In this model the atom consists of electrons which Thomson called them corpuscles surrounded by a soup of positive charge to. Thomson who discovered the electron in 1897 proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic modelIn Thomsons model the atom is composed of electrons which Thomson still called corpuscles though G. However at that time the atomic nucleus was yet to be discovered.
He is known for the Thomson atomic theory. Thomson atomic model was proposed by William Thomson in the year 1900. Thomson atomic model gives the concept of shape of the atom and proposed that the atom is spherical in shape.
In 1897 Thomson claimed the basic body of an atom is a sphere that contains electrons tiny particles within an atom that create a negative charge and a positively charged jelly around the electrons that neutralize the charge of the electrons. The model was proposed by J. The Japanese physicist Hantaro Nagaoka rejected Thomsons model.
In 1911 Rutherford showed that Thomsons model was wrong. The plum pudding atomic model or atomic theory is one of the earlier atomic theories. Thomson who invented the electron in the year 1897 suggested the atoms plum pudding model in 1904 before the atomic nucleus was found to include the electron in the atomic model.
The Early Atom Boundless Physics

Plum Pudding Atomic Model By J J Thomson Chemistrygod

J J Thomson And Ernest Rutherford History Of An Atom

Plum Pudding Model Of The Atom What Is It Who Discovered It Electrical4u

The Plum Pudding Model How A Flawed Idea Was Instrumental In Our Understanding Of The Atom
Thomson Atomic Model In Hindi Youtube
Thomson Atomic Model Plum Pudding Model Postulates Limitations
J J Thomson The History Of The Atom

Chemistry Class 9th Chapter 4 Structure Of The Atom Module Thomson S Atomic Model Youtube

Thomson Atomic Model Description Image Britannica
J J Thomson Atomic Model Ppt Video Online Download

Plum Pudding Model Of The Atom What Is It Who Discovered It Electrical4u
J J Thomson The History Of The Atom

Jj Thomson S Atomic Model And Theory Thomson S Experiment
J J Thomson Model Of An Atom Class 9 Structure Of An Atom

Thomson Atom Model And Its Drawbacks
Posting Komentar untuk "Jj Thomson Atomic Model Explanation"