Heat Of Dissolution Definition
The unit of solution enthalpy is KJmol. Enthalpies of dissolution can result in exothermic and endothermic reactions.

Exothermic Vs Endothermic Chemistry S Give And Take Discovery Express
When a solute in a solvent forms a solution it is called dissolution.
Heat of dissolution definition. Heat of dissociation definition is - the heat of reaction resulting from dissociation of molecules of a compound into smaller molecules fragments or atoms. The enthalpy change is observed when the solute is dissolved in the solvent. The amount of heatabsorbed in the formation of solutionthat contains one moleof solute.
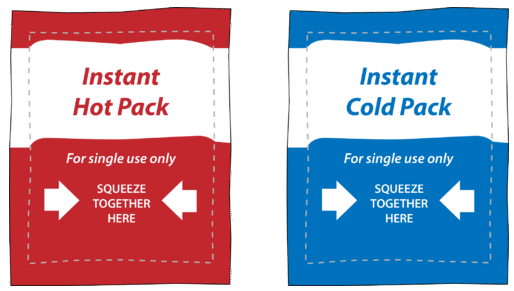
Ask Question Asked 6 years 8 months ago. Figure 1710 Chemical hot packs and cold packs work because of the heats of solution of the chemicals inside them. Search the Dictionary for More Terms.
Increasing temperature therefore decreases the solubility of the. The higher the specific heat of a substance the greater the energy change needed to change the temperature. Increasing temperature introduces more heat into the system.
Heat of Dissolution Papers The dissolution of Gibbsite and Boehmite in Bayer liquor is an endothermic heat absorbing reaction. Which formula is correct for calculating the heat of dissolution. Following Le Chateliers Principle the system will adjust to this excess heat energy by inhibiting the dissolution reaction.
The enthalpy of dissolution is commonly expressed at a common temperature in kJmol. In exothermic reactions heat energy is released when the solute dissolves in a solution. Heat Of Solution Formula The heat solution is defined as the difference in the enthalpy related to the dissolving substance in a solvent at constant pressure which is leading in infinite dilution.
Liquid water Cp 418 Jg c. Chemical hot packs and cold packs work because of the heats of solution of the chemicals inside them. I know that I can achieve.
First determine the total energy absorbed. Your source for the latest heat of dissolution definition articles. SolvationThe process of attraction and association of molecules of a solvent with molecules or ions of a solute.
The heat absorbed or released by a substance during a change in temperature depends on the specific heat the. Heat of solution definition is - the heat evolved or absorbed when a substance dissolves. Heat of solutionThe enthalpy change associated with the dissolution of a substance in a solvent at constant pressure resulting in infinite dilution.
So what this means is that it is when. A solute is the primary substance that is dissolved in a liquid called the solvent. The enthalpy of dissolution is the change in the thermodynamic potential of a substance when it is dissolved at a constant pressure in a solvent until it reaches an infinite dilution.
Definition of Heat Of Solution What is a Heat Of Solution. Active 6 years 6 months ago. The amount involved when one mole or sometimes one gram dissolves in a large excess of solvent.
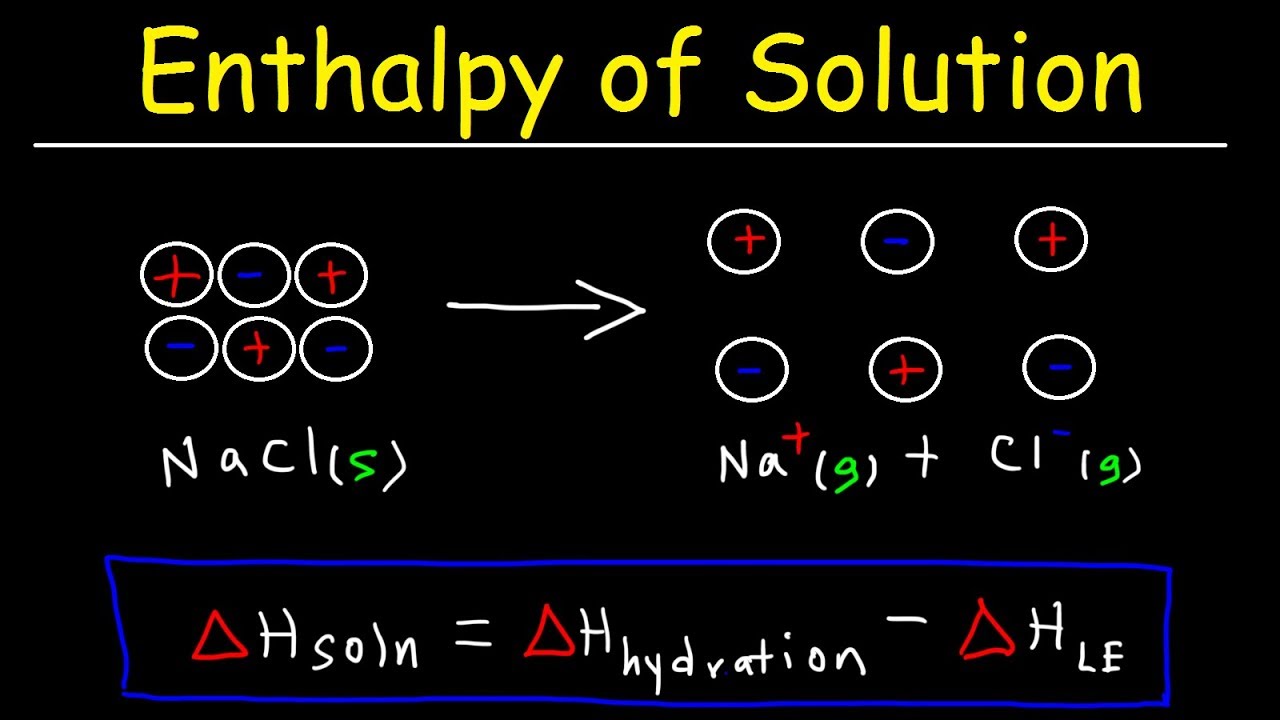
The molar heat of solution Δ H soln of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. The molar heat of solution of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. The energy absorbed by this reaction accounts for a significant fraction of the energy requirements in the Bayer process.
For calcium chloride Δ H soln 828 kJmol. Heat of Solution Example How to calculate a heat of solution. Viewed 15k times 2 begingroup I want to calculate the energy change when a solute is dissolved in water.
Heat of solution is defined as the total heat absorbed when a solute dissolves. The discovery was that inadequate replenishment of. In the summer of 1965 the assistant football coach of the University of Florida Gators requested scientists affiliated with the university study why the withering heat of Florida caused so many heat-related illnesses in football players and provide a solution to increase athletic performance and recovery post-training or game.
Heat Water has a very high specific heat. The value is positive if heatis absorbed endothermic and negative if heatis released exothermic.

Heat Of Solution Chemistry For Non Majors

Heat Of Solution Is Defined As
Molecular Basis For Water Solubility And Fat Solubility
Https Www W3spoint Com Standard Enthalpy Of Solution
Heat Of Solution Chemistry Tutorial

Enthalpy Of Solutions Study Com

15 1 Enthalpy Change Of Solution And Hydration Hl Youtube

Enthalpy Of Solution An Overview Sciencedirect Topics

Enthalpy Of Solution An Overview Sciencedirect Topics

Find The Heat Of Dissolving Delta H Dissolution Youtube
Enthalpy Of Solution Enthalpy Of Hydration Lattice Energy And Heat Of Formation Chemistry Youtube

17 13 Heat Of Solution Chemistry Libretexts
What Is The Dissolution Process In Chemistry Quora

Heat Of Solution Introduction To Chemistry

15 1 Enthalpy Change Of Solution And Hydration Hl Youtube

Posting Komentar untuk "Heat Of Dissolution Definition"