Effect Of Pressure On Viscosity Of Gases
32 Momentum transfer between the layers. Also viscosity of gases increases with temperature.
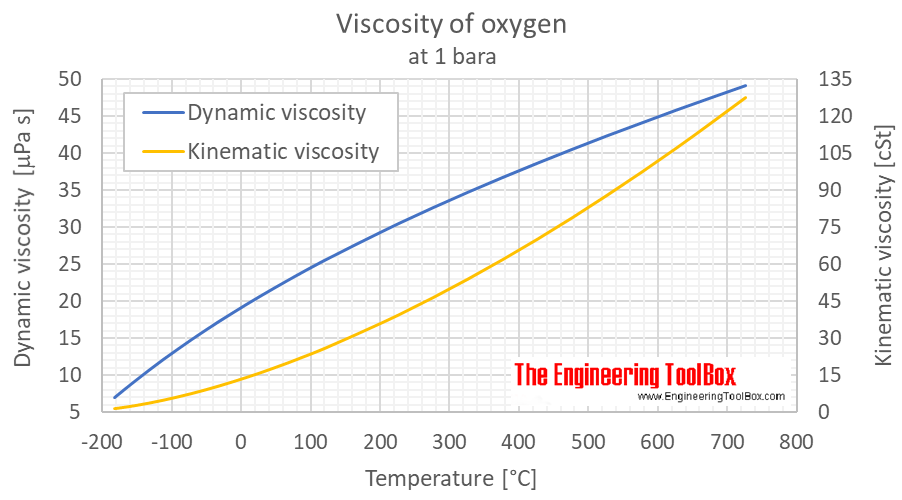
Oxygen Dynamic And Kinematic Viscosity
Due to the increase of collisions resistance between the flows of different layers also increases ie viscosity also increases.

Effect of pressure on viscosity of gases. 1 Definition of viscosity. For liquids it corresponds to the informal concept of thickness. On increasing pressure viscosity of liquid molecules increases due to the increase in the resistance to the flow of liquid.
We reanalyze the pressure dependence of viscosity of liquids of constant composition under isothermal conditions. The pressure effects on the viscosity of gases are summarized from both experimental and theoretical points of view. Pressure has an effect on both the viscosity of liquid as well as gases.
Pressure is a scalar quantity defined as force per unit area. The viscosity of a fluid is a measure of its resistance to deformation at a given rate. According to the kinetic theory of gases viscosity should be proportional to the square root of the absolute temperature in practice it increases more rapidly.
Viscosity can be conceptualized as quantifying the internal frictional force that arises between adjacent layers of fluid that are in relative motion. With high temperatures viscosity increases in gases and decreases in liquids the drag force will do the same. Viscosity will also change with pressure - but under normal conditions this change is negligible in gasses High pressure can also change the MPDFFOLect_3 viscosity of a liquid.
Articleosti_6491245 title Effect of temperature and pressure on the viscosity of steam and other gases author Farouq Ali S M abstractNote A mathematical treatment of the fluid dynamics involved in a steam-flood requires a knowledge of the viscosities of steam and mixtures of steam and hydrocarbons distilled by steam from the in-place oil for various pressures temperatures. As such pressure depends on the amount of gas in number of molecules. The textbooks seems to be talking about a limited range of P.
However for ideal gases and normal pressures it is only dependent on the temperature. For real gases thats still a very good approximation. Also asked why the viscosity decreases with increase of temperature.
Why does viscosity increase with temperature. Viscosity increases with an increase in the density of the liquid. Viscosity increases with an increase in pressure except water.
On increasing pressure the viscosity of gas molecules decreases due. Variation of Viscosity with Temperature. For most circumstances near the conditions we live in pressure doesnt have much effect on viscosity.
Pressure have effect on both viscosity of liquid as well as gases. Gases can fill a container of any size or shape. On increasing pressure the viscosity of gas molecules decreases due to the increase in glow of molecules.
The theories the generalized correlation methods and the empirical formulations are reviewed. At this link Viscosity you. Water behaves anomalously below about 30 C at low pressures increasing pressure reduces viscosity instead of increasing it.
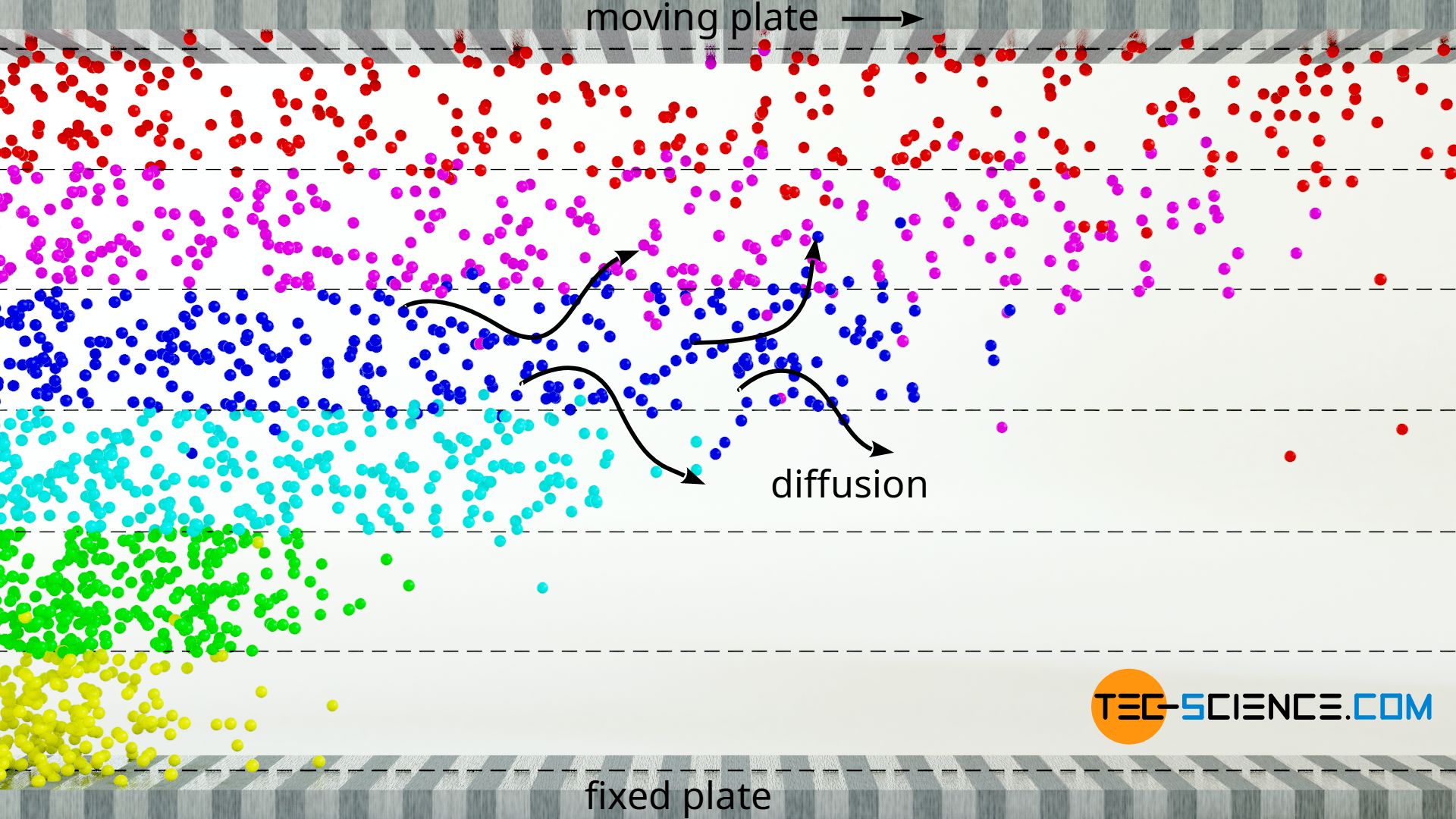
Viscosity of an ideal gas. The viscosity of a fluid is due to two contributing factors namely the cohesion between the fluid molecules and transfer of momentum between molecules. 31 Shear stress as momentum flux.
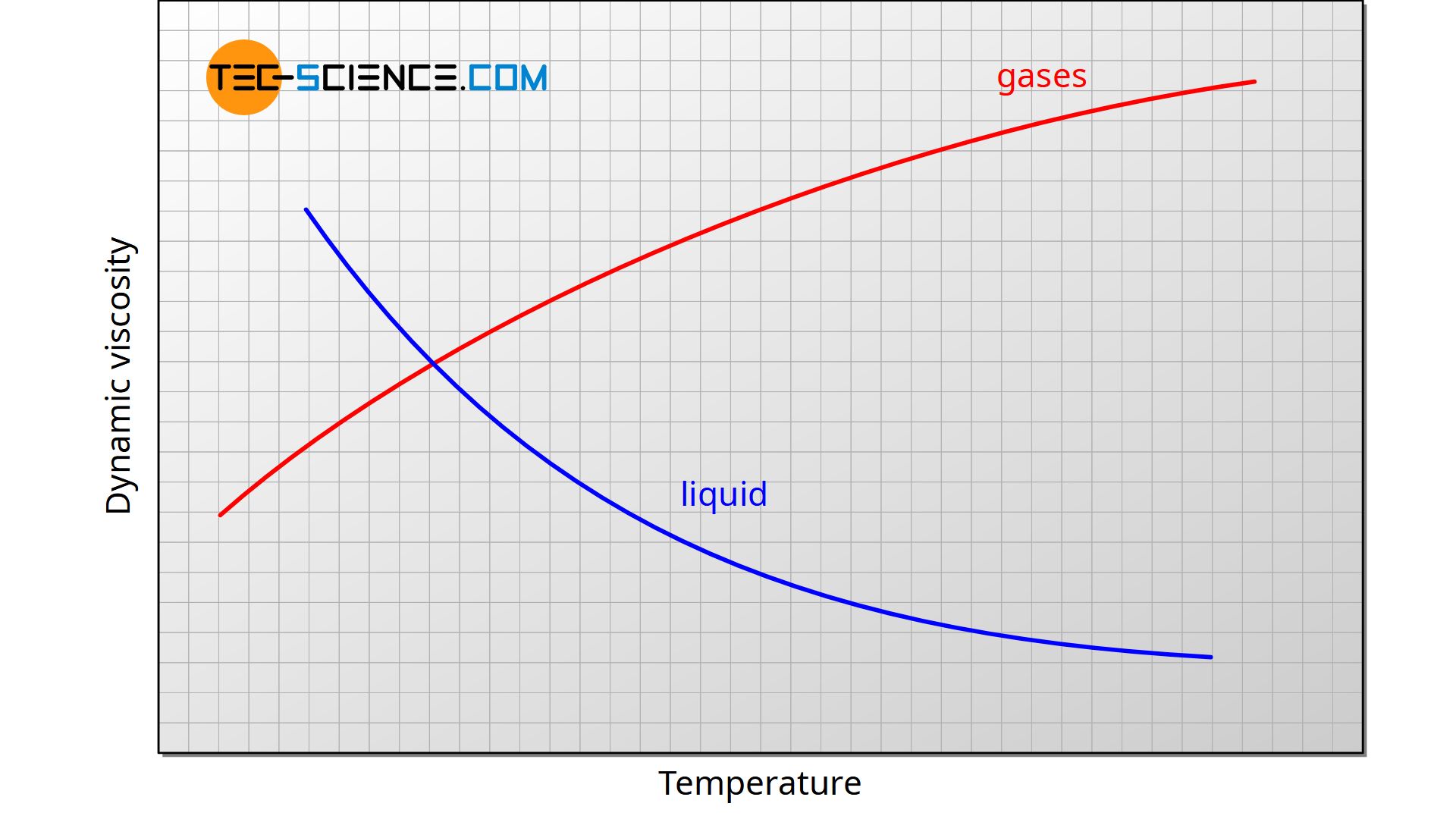
Impact of Increasing Temperature The impact of increasing temperature will be to slow down the sphere in gases and to accelerate it in liquids. Viscosity decreases with an increase in density. For example syrup has a higher viscosity than water.
The viscosity of gases is greatly altered with increasing pressure and density. 2 Viscosity of gases momentum transfer 3 Derivation of the viscosity of ideal gases. In the case of gases the interspace between the molecules is large and so the intermolecular cohesion is.
Viscosity of gases is dependent on very low or very high pressures. What is the effect of temperature on viscosity of. The gas viscosity will increase with temperature.
The result is that liquids show a reduction in viscosity with increasing temperature. As pressure increases the relative movement of molecules requires more energy hence viscosity increases. Based exclusively on very general considerations concerning the relationship between viscosity and free volume we show that at moderate values of pressure viscosity increases as a rule with increasing pressure provided the liquid is in stable or metastable.
As the pressure increases the volume decreases and the volume of these voids reduces so usually rising pressure increases the viscosity. Effect of pressure on the viscosity of pure liquids and these over only - a comparatively small range of pressure1 The effect of pressure on the viscosity of a number of lubricating oils has been recently deter mined over a range sufficient to change the viscosity many fold2 but oils are not simple substances and the results obtained with. Interesting phenomena such as the negative initial pressure dependence of the gaseous viscosity and the.
Viscosity decreases with an increase in pressure. Yes it is quite counterintuitive. On increasing pressure viscosity of liquid molecules increases due to the increase in the resistance to flow of liquid.
For ideal gases viscosity depends only on temperature. The viscosity of ideal gases is mainly based on the momentum transfer due to diffusion between the fluid layers.

Gas Viscosity An Overview Sciencedirect Topics
Viscosity Of An Ideal Gas Tec Science

Offshore Rig Monitoring A Complete Iot Solution Oil And Gas Gas Industry Iot
Viscosity Of Liquids And Gases Tec Science

How Iot Sensors Have Enriched The Oil And Gas Industry Gas Industry Oil And Gas Iot
Viscosity Of Liquids And Gases Tec Science

A Mathematical Derivation Of The Equations Relating The Pressure Temperature And Volume During An Ise In 2021 Sixth Grade Science Science Chemistry Physics Concepts
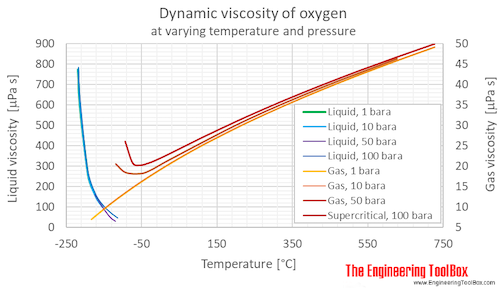
Oxygen Dynamic And Kinematic Viscosity
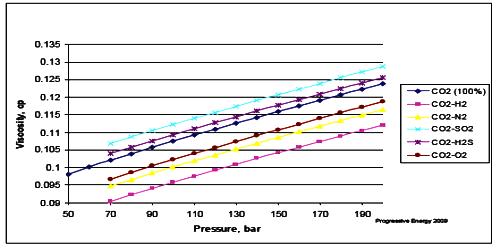
Effect Of Pressure On Viscosity Qs Study
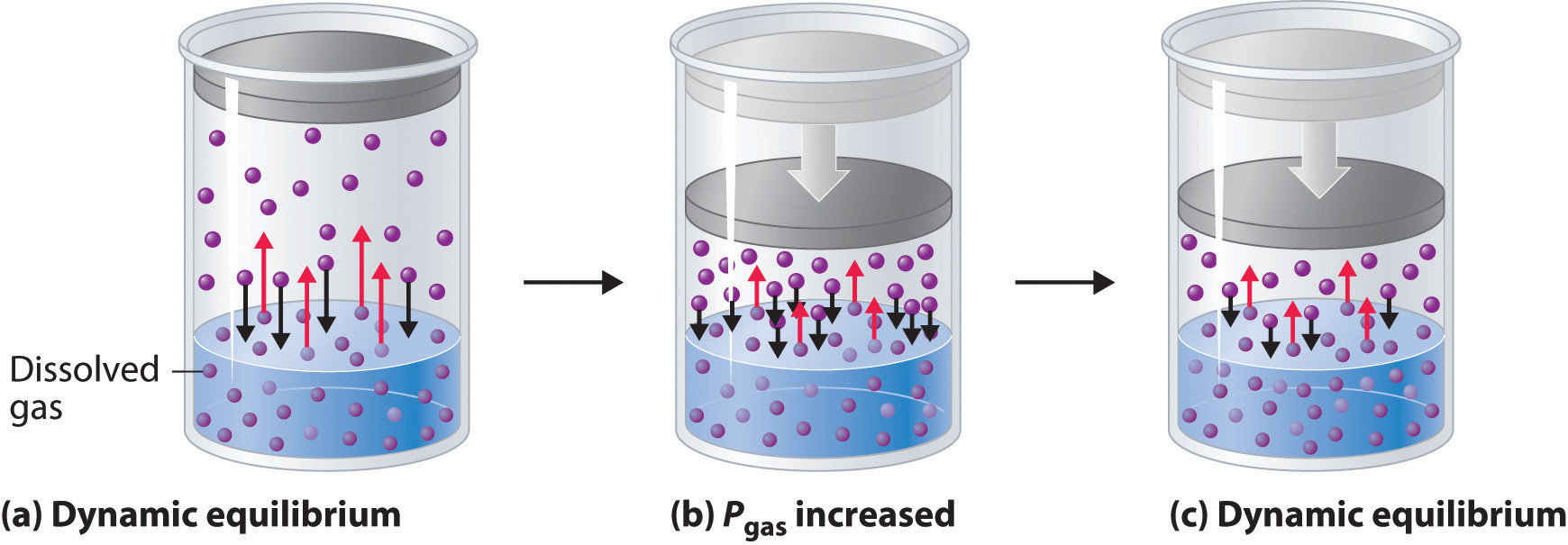
13 3 Pressure And Temperature Effects On Solubility Chemistry Libretexts

The Term Ash Content In Natural Gas Engine Oils Ngeo Oils Lubricants Gas
Effect Of Pressure On Viscosity Qs Study

Elearning Software Solution For Applied Physics Classroom Solutions Engineering Subjects Software

Learn Quiz On Pressure In Gases O Level Physics Quiz 93 To Practice Free Physics Mcqs Questions And Answers To L Physics Quiz O Levels This Or That Questions

Viscosity An Overview Sciencedirect Topics

Posting Komentar untuk "Effect Of Pressure On Viscosity Of Gases"